Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2022) Volume 13, Issue 12
Marker Assisted Selection in Groundnuts
Diriba Beyene Goonde*Received: 02-Dec-2022, Manuscript No. JPPM-22-19094; Editor assigned: 05-Dec-2022, Pre QC No. JPPM-22-19094(R); Reviewed: 19-Dec-2022, QC No. JPPM-22-19094; Revised: 26-Dec-2022, Manuscript No. JPPM-22-19094(QC); Published: 03-Jan-2023, DOI: 10.35248/2157-7471.23.13.649
Abstract
Groundnut (Arachis hypogaea L.) is an important oilseed crop worldwide. Objective of this review is to highlight molecular breeding approach such as marker assisted selection on groundnut improvement with future perspectives. The review analyzed application of marker assisted selection including simple sequence repeats, random amplified polymorphism DNAs, single nucleotide polymorphism, amplified fragment length polymorphism and inter simple sequence repeats on groundnut improvement. Among the molecular markers, random amplified polymorphic DNA is a rapid method for developing genetic maps and to determine DNA fragments to characterize peanut cultivars. DArTseq is used for SNP discovery and genotyping, which enables considerable discovery of SNPs in a wide variety of non-model organisms and provides measures of genetic divergence. Polymorphism screening performed using these newly developed SSRs will greatly increase the density of SSR markers in the peanut genetic map in the future.
Keywords
Genome; Molecular markers; Groundnut; Breeding; Improvement
INTRODUCTION
Groundnut (Arachis hypogaea L.), also known as peanut, is a member of genus Arachis and family Leguminosae [1]. Peanuts are key oilseed and food-legume crops for both humans and livestock in tropical and subtropical regions, and globally they are the fourth largest source of edible oil and third most important source of vegetable protein. Its seed contain about 50% of edible oil and the remaining 50% of the seed has high quality protein (36.4%), carbohydrate in the range 6%-24.9%, minerals and vitamin [2]. It is believed to have originated in the southern Bolivia to northern Argentina region of South America. Cultivated Groundnut (A. hypogaea L., 2n=4x=40, AABB) is self-pollinating allotetraploid legume crop belonging to the Fabaceae family [3]. Groundnut was introduced to Ethiopia by Italian explorers in the 1920s [4]. Globally China ranks first in groundnut production with 17.39 million tonnes followed by India 6.95 million tonnes, Nigeria 2.88 million tonnes, Sudan 2.88 million tonnes and Ethiopia ranks 31st with 0.129 million tonnes with national mean yield of 1.75 tons/ha, and the total area under groundnut production is 115,291 ha [5]. The most common groundnut production constraint in Ethiopia in general and the southern region, in particular, were the lack of access to improved seeds, biotic, abiotic stress, and the use of low- yielding local varieties [6,7]. Therefore, the objective of this review is to highlight molecular breeding approaches such as marker assisted selection on groundnut improvement and opportunities, challenges with future perspectives of the crop.
Status Production of Groundnut
Groundnuts are predominantly grown in developing countries (Africa and Asia where the crop finds appropriate climate for optimum production. Although, the production is concentrated in Asia (50% global area and 68% global production) and Africa (46% global area and 24% of global production) (Table 1).
| Country | Production(tons in million) | Hectare in million | Yield(kg/hectare |
|---|---|---|---|
| China | 16.685 | 4.541 | 3.674 |
| India | 6.8573 | 5.800 | 1.182 |
| Nigeria | 3.028 | 2.680 | 1.130 |
| USA | 2.578 | 0.626 | 4.118 |
| Sudan | 1.826 | 2.315 | 788.8 |
| Ethiopia | 0.129 | 74.861 | 1.731 |
Table 1: The top leading country in production and productivity of groundnut in the world [5].
Genomic Resources
Genomic resources such as molecular markers are powerful tools to characterize and harness the genetic variation present in the germplasm collection. Peanut has comparatively lower genomic resources (including transcriptome data) compared to other legumes like medicago, lotus and chickpea [8], robust molecular markers, specifically the genetic ones, as they provide the insight into the functional information. However, the available peanut high throughput transcriptome sequences are not complete; many have low N50 values, ranging from 500 to 750 bp [9].
Because peanut has such a large number of genes, it is important to have a good representation of the transcriptome. On other hand, ESTs data of cultivated peanut still remains unexplored for the development of SSR markers (Figure 1) [9].
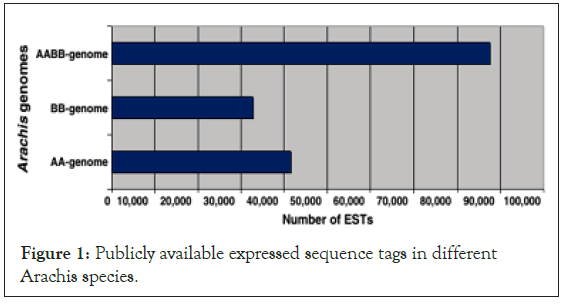
Figure 1: Publicly available expressed sequence tags in different Arachis species.
Marker Assisted Selection
Marker-assisted selection has been a plant breeding tool since it was proposed by Sax in 1923 [10]. The theory behind this method is that plant breeders could observe easy-to-score phenotypes to select difficult-to-score or low heritability traits that are linked to them. Marker assisted selection is the indirect selection of selected or desired plant phenotype depending on the closely linked DNA marker. MAS is an efficient molecular tool for breeding, in which markers linked with the desired genes are used for indirect selection for that gene in non-segregating or segregating populations. MAS is an important method for the selection of traits that are difficult, like, biotic and abiotic stress tolerance in a crop [11]. Compared with conventional phenotypic selection, MAS is not influenced by environmental conditions because it detects the structural polymorphisms at the molecular level. Further MAS is cheaper and less labour intensive, allows selection in off-season nurseries and has a potential to accelerate the breeding process [12].
Molecular markers among all genomic resources, molecular markers have direct use for germplasm characterization, trait mapping and molecular breeding. Several marker systems have been developed during the last three decades. For instance Restriction Fragment Length Polymorphisms (RFLPs), Random Amplified Polymorphic DNAs (RAPDs), Amplified Fragment Length Polymorphisms (AFLPs) and Diversity Arrays Technology (DArT) markers have proved their utility from time to time [13]. However, Simple Sequence Repeats (SSRs) or microsatellites and Single Nucleotide Polymorphism (SNP) markers are generally preferred for plant genetics and breeding applications. While SSR markers are multi-allelic, co-dominant and easy to use, the SNP markers are highly amenable to high-throughput genotyping approaches. Development and application of SNP markers, however, is still not routine in crop species and especially not in low-tech laboratories (Table 2).
| Molecular markers | Type | Amplification of markers/techniques used for the identification |
|---|---|---|
| Restriction fragments length polymorphism | Co-dominant | Depends on point of mutation |
| Sequence characterised amplified region | Co-dominant | Depends on mutation at primer annealing site in the specific region of DNA stand |
| Amplified fragments length polymorphism | Dominant | Depends on mutation at primer annealing site in the target DNA and change restriction site in the target |
| Sample sequence repeats | Co-dominant | Difference in the number of repeats of motif |
| Diversity arrays technology | Dominant | micro array hybridization, they produce from genomic libraries through amplification of candidate or random clones |
| Single nucleotide polymorphism | Co-dominant | Point mutation in the target sequence information |
| Sequence characterized region | Co-dominant | Depend on mutation at primer annealing site in specific region of DNA strand |
Table 2: Commonly used molecular markers.
Markers for Target Traits
The approach of identifying markers for targets traits swiftly changed with the development of linkage maps in groundnut [14]. Seed weight is controlled by a combination seed features such as seed length, seed width, and seed thickness. Several genes for seed- related traits have been obtained in many crops using the forward genetic strategy and reverse genetic strategies [15]. QTL analysis was used for identification of QTLs for several important traits such as drought tolerance related traits, resistance to foliar disease and nutritional quality traits (Table 3) [16-23].
| Population | Traits | Markers |
|---|---|---|
| Yuanza9102 × ICGV 86699 | Rust resistance | AFLP |
| TAG 24 × GPBD 4 | Rust resistance | SSR |
| TAG 24 × GPBD 4, TG 26 × GPBD 4 |
LLS rust resistance | SSR |
| Zhonghua 5 × J 11 | Afloxin contamination | AFLP |
| TAG 24 × ICGV 86031 | Drought tolerance | SSR |
| Tamrun OL01 × BSS 56 | Pod and kernel traits | SSR |
| TG 26 × GPBD 4 | protein content | SSR |
| TG 26 × GPBD 4 | Oil content | SSR |
| Germplasm accessions and breeding lines | High oleic acid content (FAD2A) | real time- Pcr |
| Archis hypogaea × TxAg-M | Arenaria resistance | RFLP |
| Yuanza 9102 × chico | Bacterial wilt resistance | SSR |
Note: RAPD: Randomly Amplified Polymorphic DNA; RFLP: Restriction Fragment Length Polymorphism; SSR, Simple Sequence Repeat; AFLP: Amplified Fragment Length Polymorphism; AS-PCR: Allele Specific Polymerase Chain Reaction; FAD: Fatty Acid Desaturase; ELS: Early Leaf Spot; LLS: Late Leaf Spot.
Table 3: Molecular markers associated with trait specific genes/QTLs in groundnut.
Molecular Markers in Groundnut
Cultivated groundnut has been analyzed by several markers systems including RFLPs, RAPDs, AFLPs and SSRs. Restriction Fragment Length Polymorphism (RFLPs) represented the first marker system that had a large number of polymorphisms. They are used widely to create linkage maps and to implement indirect selection strategies. In A. hypogaea, little molecular variation has been detected by using RFLP technologies. They have been used to analyze species in section Arachis (representing taxa that will hybridize with A. hypogaea) and clusters that formed using multivariate analyses [24] correspond closely with morphological groups tetraploids were clearly separated from diploids in both investigations [25,26]. Utilized RFLPs to examine genetic diversity among 18 accessions of A. duranensis Krapov and W.C. Gregory and found a large amount of variation in the species. Individual accessions also could be uniquely identified by RFLP patterns. The cultivated peanut resulted from a cross between A. duranensis and A. ipaensis Krapov and W.C. Gregory, and chloroplast analysis indicated that A. duranensis was the female progenitor of the cross. An RFLP map was developed for peanut by analyzing an F2 population from the diploid (2n=2x=20) interspecific cross of A. stenosperma Krapov and W.C. Gregory (ace, HLK 410) and A. cardenasii Krapov and W.C. Gregory (ace, GKP 10017). The linkage map covered 1063 cM with 117 markers in 11 linkage groups [16]. Fifteen unassociated markers also were reported. A second molecular map of peanut was created by Burow, Patterson, and Simpson using the tetraploid cross Florunner X 4x (A. batizocoi Krapov. and W.C. Gregory (A. cardenasii × A. diogoi Hoehne) Burow (pers. commun.). Most of the 380 RFLP markers that have been mapped had disomic inheritance, with the exception of one linkage group which may be polysomic.
Simple Sequence Repeats
Simple Sequence Repeats (SSRs) are genomic fragments that consist of randomly repeated units that are present in both coding and non-coding regions of the genome [17]. SSR markers, designed by flanking sequences, are useful for and widely applied in plant genetic analyses and marker-assisted selection breeding. Currently, g-SSR markers are common and popular for such analyses, and they have wide applications in molecular genetics and breeding, because they have multiple advantages, such as simplicity, abundance, ubiquity, variation, co-dominance, and multi-allelism [17]. Even though the peanut genome had not yet been resolved. With the recent completion of genome sequencing of peanut and two diploid progenitor species, A. duranensis and A. ipaensis, a large number of genome-wide g-SSRs were identified. SSR markers linked to resistance to early leaf spot, groundnut rosette disease, and rust and aflatoxin contamination across African cultivated groundnut varieties were identified suitable parents for mapping populations or breeding [18]. Genotypes with similar genetic backgrounds tended to cluster in the same subgroup, indicating the effectiveness of SNP markers in assigning the tested genotypes into homogenous groups [19]. Simple Sequence Repeat (SSR) alleles associated with agronomic traits in at least two environments. These markers were further investigated for their potential use in genetic studies by ascertaining their genetic diversity in the natural population.
Inter Simple Sequence Repeats
Inter Simple Sequence Repeats (ISSR) marker has been reported as a rapid, reproducible, and cheap fingerprinting technique based on the variation found in the regions between microsatellites. It is a fast, inexpensive genotyping technique based on variation in the regions between microsatellites (Inter Simple Sequence Repeats analyses offer breeders and geneticists with competent means to link phenotypic and genotypic variations in various fields of plant improvement) [20].
Randomly Amplified Polymorphic DNA Markers
Among the molecular markers, Random Amplified Polymorphic DNA (RAPD) is a rapid method for developing genetic maps and to determine DNA fragments to characterize peanut cultivars. PCR based Randomly Amplified Polymorphic DNA markers are good genetic markers because they give rapid results, economically convenient and use small oligonucleotide primers. With a small quantity of template, a very large number of fragments are generated from different regions of the genome and hence, multiple loci may be examined very quickly [23].
Diversity Arrays Technology
Diversity Arrays Technology (DArT), which is based on genome complexity reduction and SNP detection through hybridization of PCR fragments, has been used in Genome-Wide Association Studies (GWAS), construction of dense linkage maps and mapping quantitative trait loci (Table 4) [21]. SSR, simple sequence repeat markers, TEM, Transposable element markers, RAPD, random amplified polymorphic DNA. Source: [6,7].
| Marker name | Marker type | Marker sequence | Co-dominant |
|---|---|---|---|
| Forward primer | Reverse primer | ||
| IPAHM103 | SSR | GCATTCACCACCATAGTCCA | TCCTCTAGCTTTCCTCCATCA |
| GM1536 | SSR | AAAGCCCTAGAAAAGAAAGCAG | ATGCATTTGCAGGTTCTGGT |
| GM2301 | SSR | GTAACCACAGCTGGCATGAAC | CTTCAAGAACCCACCAACAC |
| GM2079 | SSR | GGCCAAGGAGAAGAAGAAAGA | GAAGGAGTAGTGGTGCTGCTG |
| GM1991 | SSR | GAAAATGATGCCGAGAAATGT | GGGGAGAGTGCAGAAAGAGA |
| TE360 | TEM | GGATATGATGCCCATAGCTGA | TGCTGACTACTTGCAATGCC |
| TE498 | TEM | ATGACTTACATGTAGCAATTG | TGAAAGGAGTCAAAGGTCATG |
| S197 | RAPD | CTGTCGAACCATGGAAGAAGATCC | CCAACTTGATGGTAGAAGTATGCEGCTT |
| AHCW0061 | SSR | TCATGTGAATTTGTGGACGGT | CCAGGTTTTTGAGGTCCCTGA |
| AHCW0310 | SSR | GTTCAAGGGCTGTGCATTGG | GGGTTCGACTCCCGTCTTAT |
| AHCW0545 | SSR | ACAGAAGAAGAAACAGCGCG | TTCCGTCATGTGCTTCGGAA |
| AHCW0618 | SSR | AAATTTGAGCACGCAATCCCC | TGTCTTTTTCCTCGCCTTTGT |
| AHCW0700 | SSR | TGGAAGTTTCACGGGACAGG | GTAGCAAGCTTCCCCACCAT |
| AHCW0768 | SSR | GGACCCATTTTGCAAGAGAGA | CGGATTGCAACATTGGCGAA |
| AHCW1250 | SSR | ACAGCTGCTCTTCTCTGTG | CCCACTCAAAATCGGATTTGGA |
| AHCW1510 | SSR | TCCTGCACCATGACCATGAA | TGTTCGGCACCAATCTGTCA |
| AHCW1765 | SSR | CGCTGGTCTGGCATTTAACG | AAGGGAGGAGGAGTTGGGTT |
| AHCW1862 | SSR | TGTTCAGGGAGTTGTTTGGACT | GGGCAAGCTCTTTAAACTGCA |
Table 4: Some molecular marker systems developed for genetic analysis and breeding in groundnut.
Perspectives
Molecular markers can assist in the selection process with phenotypic selection and speed up the pace of breeding cycle, in recent time’s technologies such as next generation sequencing i.e., low with high throughput, Genome Selection and Genotype by sequencing can be used to achieve the desired goal in molecular breeding approaches. The genome/gene space sequence would provide the opportunities to link the phenotype with genes. The future of peanut genomics and use of molecular tools in breeding seems to be bright that will ensure the peanut improvement for different production as well as quality constraints.
Discussion and Conclusion
Molecular markers are used to identify quantitative trait loci which enhance the efficiency of selecting complex trait in plant breeding. MAS is an efficient molecular tool for breeding, in which markers linked with the desired genes are used for indirect selection for that gene in non-segregating or segregating populations. Now a days, DArT, SSR, SNP, ISSR, etc. with high throughput technologies are very exciting markers, which enhances the crop with desired traits and induces tolerance against biotic and abiotic stresses in a short period of time. Molecular markers can provide information that can help define the distinctiveness of species and their ranking according to the number of close relatives and their ranking according to the phylogenetic position. RAPD patterns generated from peanut cultivars could be used as genomic fingerprint to establish the identity of a given genotype. The utilization of DArT marker system may limit efficient genetic analysis of groundnut genetic resources for cultivar development. Development of highly discriminative and informative DArT markers is useful for genetic analysis and breeding in groundnut. A desirable molecular marker should have high polymorphism, frequent occurrence, should be easy to use and should be quick, co-dominant inheritance, equally dispersed all over the genome, high transferability and reproducibility, less expensive and phenotypically neutral. However, it will still take some time before cost-effective SNP genotyping platforms are available for genotyping the tetraploid peanut germplasm collections or peanut mapping populations. Extension of SNP-based maps to the tetraploid has not been accomplished yet, and will require separation of A and B-genome sequences, but is expected to greatly accelerate genetic mapping and marker- assisted selection.
Acknowledgements
Wallaga University and Haramaya University Fully acknowledged for giving chance to Fellow Doctor of Philosophy. Seltene Abady (PhD) is sincerely acknowledged for guiding me the review.
References
- Baraker B, Jha SK, Wani SP, Garg KK. Effect of balanced fertilizer management practices on factor of productivity on Groundnut (Arachis hypogaea L.) cultivation. Int J Chem Stud. 2017;5(4):1288-1291
- Janila P, Nigam SN, Pandey MK, Nagesh P, Varshney RK. Groundnut improvement: Use of genetic and genomic tools. Front Plant Sci. 2013;4:1-16
[Crossref] [Google Scholar] [PubMed]
- Wakjira A. Groundnut breeding activities in Ethiopia. In: Paper Presented at the First National Oilseeds Work Shop.1991.
- FAO. Food and Agriculture Organization of the United Nations. 2021.
- Abady S, Shimelis H, Janila P. Farmers’ perceived constraints to groundnut production, their variety choice and preferred traits in eastern Ethiopia: Implications for drought-tolerance breeding. J Crop Improv. 2019;33:1-17.
- Abady S, Shimelis H, Janila P, Mashilo J. Groundnut (Arachishypogaea L.) improvement in sub-Saharan Africa: A review. Acta Agric Scand B Soil Plant Sci. 2019; 69(6):528-545.
- Sato S, Nakamura Y, Kaneko T, Asamizu E, Kato T, Nakao M, et al. Genome structure of the legume, Lotus japonicus. Genome Res. 2008;15:227-239.
[Crossref] [Google Scholar] [PubMed]
- Guimaraes PM. Global transcriptome analysis of two wild relatives of peanut under drought and fungi infection. BMC Genomics. 2012;13:387.
[Crossref] [Google Scholar] [PubMed]
- Arus P, Moreno-Gonzalez J. Hayward MD, Bosemark NO, Romagosa I, editors. Plant Breeding: Principles and Prospects. 1993;314-331.
- Das G, Patra JK, Baek KH. Insight into MAS: 2017 a molecular tool for development of stress resistant and quality of rice through gene stacking. Front Plant Sci. 2017;13(8):985.
[Crossref] [Google Scholar] [PubMed]
- Kumpatla SP, Buyyarapu R, Abdurakhmonov IY, Mammadov JA. Genomics-assisted plant breeding in the 21st Century. In: Abdurakhmonov I, editor. Technological Advances and Progress. 2012;132-184.
- Varshney RK, Hoisington DA, Tyagi AK. Advances in cereal genomics and applications in crop breeding. Trends Biotechnol. 2006;24(11):490-499
[Crossref] [Google Scholar] [PubMed]
- Pandey M. Advances in Arachis genomics for peanut improvement. Biotechnol Adv. 2012;30:639-651.
[Crossref] [Google Scholar] [PubMed]
- Si L, Chen J, Huang X, Gong H, Luo J, Hou Q, et al. OsSPL13 controls grain size in cultivated rice. Nat Genet. 2016;48:447-456.
[Crossref] [Google Scholar] [PubMed]
- Halward TM, Stalker HT, Kochert G. Development of an RFLP linkage map indiploid peanut species. Theoretical and Applied Genetics.1993;87(3):379-384.
[Crossref] [Google Scholar] [PubMed]
- Haq SU, Jain R, Sharma M, Kachhwaha S, Kothari SL. Identification and characterization of microsatellites in expressed sequence tags and their cross transferability in different plants. Int J Genomics. 2014;2014:863948.
[Crossref] [Google Scholar] [PubMed]
- Kanyika BTN, Lungu D, Mweetwa AM, Kaimoyo E, Njung'e VM, Monyo ES, et al. Identification of groundnut (Arachis hypogaea) SSR markers suitable for multiple resistance traits QTL mapping in African germplasm. Electron J Biotechnol. 2015;18:61-67.
- Adu BG, Badu-Apraku B, Akromah R, Garcia-Oliveira AL, Awuku FJ, Gedil M. Genetic diversity and population structure of earlymaturing tropical maize inbred lines 146 using SNP markers. 2019;14(4):e0214810. Journal Pone.
[Crossref] [Google Scholar] [PubMed]
- Tadele S, Mekbib F, Tesfaye K. Genetic diversity of coffee (Coffea arabica L.) landraces from Southern Ethiopia as revealed by inter simple sequence repeat marker. J Agric Res.2014;3(1):024-034.
- Abu Zaitoun SY, Jamous RM, Shtaya MJ, Mallah OB, Eid IS, Ali-Shtayeh MS. Characterizing palestinian snake melon (Cucumis Melo Var. Flexuosus) germplasm diversity and structure using SNP and DArTseq markers. BMC Plant Biol. 2018;18:246.
[Crossref] [Google Scholar] [PubMed]
- Edae EA, Byrne PF, Haley SD, Lopes MS, Reynolds MP. Genome-wide association mapping of yield and yield components of spring wheat under contrasting moisture regimes. Theor Appl Genet. 2014;127:791-807.
[Crossref] [Google Scholar] [PubMed]
- Kumar A, Jain S, Elias EM, Ibrahim M, Sharma LK. An overview of QTL identification and marker-assisted selection for grain protein content in wheat. Agro Biological. 2018;245-274.
- Kochert G, Halward TM, Branch WD, Simpson CE. RFLP variability in peanut cultivars and wild species. Theor App Genet. 1991;81:565-570.
[Crossref] [Google Scholar] [PubMed]
- Stalker HT, Simpson CE, Pattee HE, Stalker HT, Genetic resources of wild Arachis and genetic diversity. Adv Peanut Sci. 1995;14-53.
- Stalker HT. A morphological appraisal of wild species in section Arachis of peanuts. Peanut Sci. 1990;17:117-122.
- Kochert G, Stalker HT, Ginenes M, Galgaro L, Moore K. The genome sequence of segmental allotetraploid peanut Arachis hypogaea). Amer J Bot. 1996;83:1282-129l.
Citation: Goonde DB (2023) Marker Assisted Selection in Groundnuts. J Plant Pathol Microbiol. 13:649.
Copyright: © 2023 Goonde DB. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

