Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Short Communication - (2024) Volume 12, Issue 2
Lipoprotein(a) is a Prevalent yet Vastly Underrecognized Risk Factor for Cardiovascular Disease
Jacob Rendler, Mia Murphy and Calvin Yeang*Received: 09-Feb-2024, Manuscript No. HCCR-24-24887; Editor assigned: 12-Feb-2024, Pre QC No. HCCR-24-24887 (PQ); Reviewed: 26-Feb-2024, QC No. HCCR-24-24887; Revised: 04-Mar-2024, Manuscript No. HCCR-24-24887 (R); Published: 12-Mar-2024, DOI: 10.35248/2375-4273.24.12.397
Description
Lipoprotein(a) (Lp(a)), consisting of an apoB containing lipoprotein with similarities to low density lipoprotein (LDL) covalently bound to apolipoprotein(a) [apo(a)], is a common cardiovascular disease (CVD) risk factor with elevated levels present in 10-20% of the population [1-3]. The composition of Lp(a) has informed the mechanisms by which it mediates CVD. Lp(a) is the major lipoprotein carrier of pro-inflammatory and pro-calcific oxidized phospholipids, shares pro-atherogenic features with other cholesterol rich apoB lipoproteins, and additionally may be pro- thrombotic [4,5]. An established and expanding body of high quality epidemiological and human genetic evidence base supports a causal role of Lp(a) as a risk factor for CVD including coronary and peripheral arterial disease and ischemic stroke, calcific aortic valve disease, and atrial fibrillation (Figure 1), and has reviewed in recent scientific statements by the American Heart Association (AHA) and European Atherosclerosis Society (EAS) [6,7].
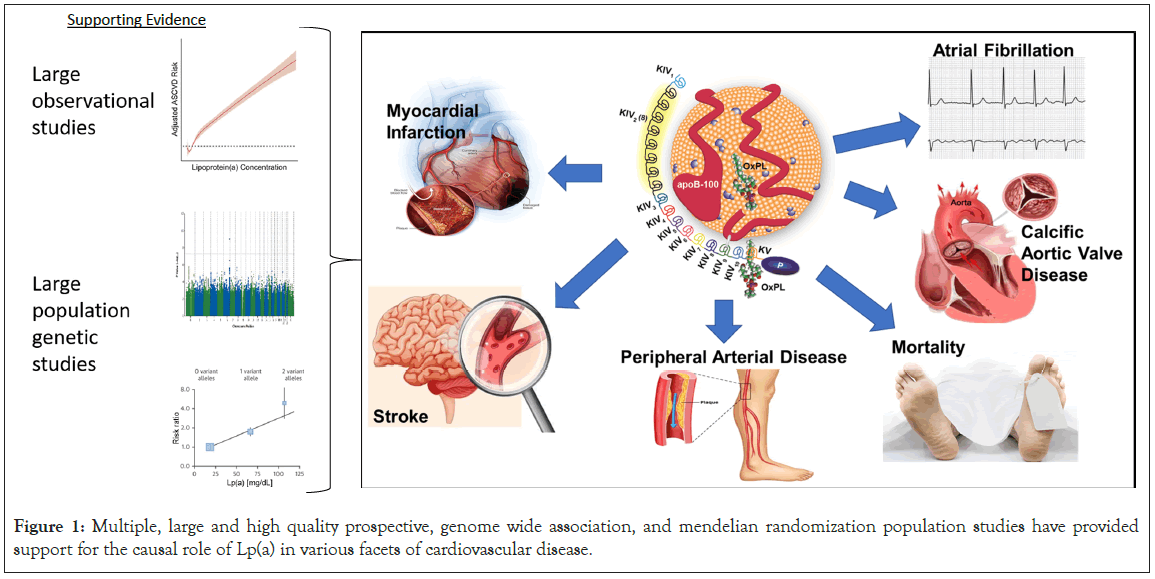
Figure 1: Multiple, large and high quality prospective, genome wide association, and mendelian randomization population studies have provided support for the causal role of Lp(a) in various facets of cardiovascular disease.
Knowledge of Lp(a) levels informs comprehensive CVD risk assessment and international medical societies including the AHA, European Society of Cardiology (ESC), National Lipid Association (NLA), American Association of Clinical Endocrinology (AACE), Canadian Cardiovascular Society (CCS), and EAS have provided guidance on Lp(a) testing (Table 1). There is consensus between these organizations supporting Lp(a) testing in individuals with a family or personal history of premature CVD. Moreover, the ESC, EAS, and CCS recommend Lp(a) screening in all adult individuals to identify those with elevated levels contributing to higher CVD risk. As Lp(a) levels are ~ 90% genetically determined with only modest potential fluctuations influenced by age, lifestyle, and co-morbidities, a single test would be sufficient to identify or exclude individuals with elevated Lp(a) levels >50 mg/dL (125 nmol/L) associated with increased CVD risk [6,7].
Despite a 10%-20% prevalence of elevated Lp(a), utilization of Lp(a) testing in clinical practice has been long felt to be poor. Recently, Lp(a) testing rates have been systematically quantified confirming prior anecdotal suspicions. A study consisting of >5.5 million adults across 6 academic health systems in California from 2013-2021 revealed only 0.3% of the population had Lp(a) testing (Figure 2) [8]. Of the patients that had been screened for CVD risk factors, identified by having at least one lipid panel, just 1.8% also had Lp(a) testing. Only less than 4% of individuals with either a personal or family history of CVD had Lp(a) testing. Similarly low rates of Lp(a) utilization were reported in other health systems across the US, with 0.06%-3% of individuals tested [9,10]. Moreover, gender and racial disparities in Lp(a) testing rates exist–more likely in men compared to women and whites compared to blacks [8].
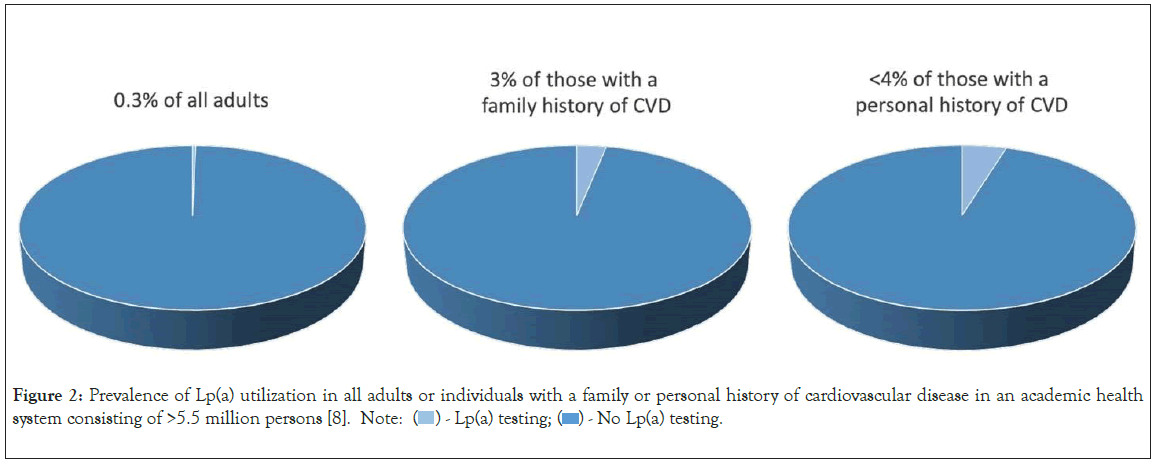
Figure 2: Prevalence of Lp(a) utilization in all adults or individuals with a family or personal history of cardiovascular disease in an academic health system consisting of >5.5 million persons [8]. Note:  - No Lp(a) testing.
- No Lp(a) testing.
Why is Lp(a) so infrequently tested despite being one of the most common CVD risk factors? There have been several reasons suggested anecdotally. These include an underrecognition of the evidence base supporting Lp(a) as a causal CVD risk factor and/ or guideline indications for Lp(a) testing. Clinicians may not be fully aware of existing guidance on how to incorporate Lp(a) into CVD risk assessment and modification for their patients and instead operate with the assumption that Lp(a) testing would not change clinical management. While CVD outcomes trials with specific and potent Lp(a) lowering therapies are ongoing, some clinicians may feel that readout of these trials are required before changing practice patterns around Lp(a) testing. Additionally, clinical laboratories vary in their choice of calibrators for Lp(a) with some reporting values in mg/dL and others in nmol/L. The lack of standardization for Lp(a) measurements may be a barrier for testing due to confusion with interpretation or perceived imprecision of currently available assays. Lastly, concerns around testing cost to the patient, insurance, and healthy system may influence Lp(a) utilization. The prevalence of these barriers, and their order of importance to clinicians will need to be objectively and systematically evaluated to fully understand and lead to action to improve Lp(a) utilization.
It is important to note that many, if not all of these potential barriers to Lp(a) testing can be overcome by more awareness of existing knowledge. Dissemination of the existing guidance for Lp(a) testing as well as risk assessment and modification for patients with elevated Lp(a) at local and national continuing medical education (CME) events attended by primary care physicians, cardiologists, vascular specialists, lipid specialists, and stroke neurologists is one opportunity to empower clinicians to check Lp(a) when appropriate. Multiple ongoing clinical trials evaluating targeted therapies that potently lower Lp(a) by 25%-100% (Table 2), fuel potential that additional risk modifying options may be soon available for patients with elevated Lp(a). However, recognition of patients with elevated Lp(a) through testing is potential for enrolling adequately powered trials and the confidence in interpreting their results. Regarding Lp(a) assays, efforts to standardize Lp(a) measurements in nmol/L are ongoing [11], however, routinely available clinical assays are sufficient for identification of patients with elevated Lp(a) levels–either >50 mg/dL or >125 nmol/L. Lastly, Lp(a) testing is relatively inexpensive and cost effective. Lp(a) tests are simple immunoassays with cost to insurance or patients ranging from $21-$99 (Table 3). As Lp(a) levels are predominately genetically determined, a single Lp(a) test would sufficiently determine Lp(a) attributable risk in most individuals.
 |
 |
 |
 |
 |
 |
|
|---|---|---|---|---|---|---|
| 2018 | 2019 | 2019 | 2020 | 2021 | 2022 | |
| All individuals to identify those at high CVD risk | X | X | X | |||
| Family/personal history of premature ASCVD | X | X | X | X | X | X |
| Specific Indications | To aid in decision making for statins in those with intermediate ASCVD risk | Individuals with south Asian or African ancestry, Individuals with a 10 year ASCVD risk ≥ 10% | Youth with a history of ischemic stroke and no other identifiable risk factors. |
|||
| To identify a possible cause for less than anticipated pharmacologic LDL-C lowering | Patients with statin resistance | |||||
| To identify those at risk for progressive aortic stenosis | Patients with a personal or family history of aortic stenosis | |||||
Table 1: Recommendations for Lp(a) testing by major international clinical guidelines and expert consensus statements. American Heart Association (AHA)/American College of Cardiology (ACC) [12], European Society of Cardiology (ESC) [13], National Lipid Association (NLA) [14], American Association of Clinical Endocrinology (AACE) [15], Canadian Cardiovascular Society (CCS) [16], European Atherosclerosis Society (EAS) [7].
| Therapy | Mechanism of action | Modification | % Lp(a) reduction | Current clinical trial stage |
|---|---|---|---|---|
| Muvalaplin | Inhibits the assembly of Lp(a) | ~25-65% [17] | Phase 1 | |
| Pelacarsen | AntiSense Oligonucleotide | N-acetyl galactosamine | 35-80% [18] | Phase 3 |
| (ASO) | (GalNAc) | |||
| Olpasiran | Small interfering RNA (siRNA) | GalNAc | 70-100% [19] | Phase 3 |
| SLN360 | siRNA | GalNAc | 46-98% [20] | Phase 2 |
| Lepodisiran | siRNA | GalNAc | 41-97% [21] | Phase 2 |
Table 2: Emerging targeted Lp(a) lowering therapies.
| Laboratory name | Lp(a) calibrator units | Cost |
|---|---|---|
| Labcorp | nmol/L | $49 |
| Quest | nmol/L | $91 |
| Boston heart diagnostics | nmol/L | $21 |
| empowerDX | mg/dL | $99 |
| Letsgetchecked | mg/dL | $89 |
| Healthlabs | nmol/L | $49 |
Table 3: Lp(a) testing costs at select clinical laboratories.
Conclusion
Lp(a) testing is essential for comprehensive CVD risk evaluation and is indicated in those with a personal or family history of premature CVD, if not all individuals. There is an urgent need to systematically understand the barriers around Lp(a) utilization. A survey based assessment of awareness, beliefs and practice patterns with Lp(a) testing broadly amongst primary care clinicians, cardiologists, lipidologists, stroke neurologists, vascular specialist, population health, and payors is now needed to guide practice changing education and implementation to improve Lp(a) testing.
Disclosures
CY receives consulting fees from Kaneka Corporation. The other authors have no relevant disclosures.
Funding
CY receives research support from NIH grant 1K08HL150271.
References
- Nordestgaard BG, Langsted A. Lipoprotein(a) as a cause of cardiovascular disease: Insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57(11):1953–1975.
[Crossref] [Google Scholar] [PubMed]
- Varvel S, McConnell JP, Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532 359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36(11):2239–2245.
[Crossref] [Google Scholar] [PubMed].
- Patel AP, Wang M, Pirruccello JP, Ellinor PT, Ng K, Kathiresan S, et al. Lp (a)(lipoprotein [a]) concentrations and incident atherosclerotic cardiovascular disease: New insights from a large national biobank. Arterioscler Thromb Vasc Biol. 2021;41(1):465–474.
- Boffa MB, Koschinsky ML. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol. 2019;16(5):305–318.
[Crossref] [Google Scholar] [PubMed]
- Yeang C, Wilkinson MJ, Tsimikas S. Lipoprotein(a) and oxidized phospholipids in calcific aortic valve stenosis. Curr. Opin. Cardiol. 2016;31(4):440–450.
[Crossref] [Google Scholar] [PubMed]
- Reyes-Soffer G, Ginsberg HN, Berglund L, Duell PB, Heffron SP, Kamstrup PR, et al. Lipoprotein(a): A genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: A scientific statement from the American heart association. Arterioscler Thromb Vasc Biol. 2022;42(1):e48-60.
[Crossref] [Google Scholar] [PubMed]
- Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43(39):3925–3946.
[Crossref] [Google Scholar] [PubMed]
- Bhatia HS, Hurst S, Desai P, Zhu W, Yeang C. Lipoprotein(a) testing trends in a large academic health system in the United States. J Am Heart Assoc. 2023;19;12(18):e031255.
[Crossref] [Google Scholar] [PubMed]
- McGowan M, Wilemon K, Ahmed C, Myers K, MacDougall D. Characterization of lipoprotein(a) measurement in a large US healthcare dataset. J Clin Lipidol. 2022;16(3):e36–e37.
- Kelsey MD, Mulder H, Chiswell K, Lampron ZM, Nilles E, Kulinski JP, et al. Contemporary patterns of lipoprotein(a) testing and associated clinical care and outcomes. Am J Prev Cardiol. 2023;14:100478.
[Crossref] [Google Scholar] [PubMed]
- Standardization for lipoprotein(a) Measurement in Humans | NHLBI, NIH. 2019.
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American college of cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082-143.
[Crossref] [Google Scholar] [PubMed]
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41(1):111–188.
- Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, et al. Use of lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the national lipid association. Don P. Wilson, MD, on behalf of the writing group. J Clin Lipidol. 2019;16(5):77-95
[Crossref] [Google Scholar] [PubMed]
- Handelsman Y, Jellinger PS, Guerin CK, Bloomgarden ZT, Brinton EA, Budoff MJ, et al. Consensus statement by the American association of clinical endocrinologists and American college of endocrinology on the management of dyslipidemia and prevention of cardiovascular disease algorithm - 2020 executive summary. Endocr Pract. 2020;26(10):1196–1224.
[Crossref] [Google Scholar] [PubMed]
- Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, et al. Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37(8):1129–1150.
[Crossref] [Google Scholar] [PubMed]
- Nicholls SJ, Nissen SE, Fleming C, Urva S, Suico J, Berg PH, et al. Muvalaplin, an oral small molecule inhibitor of lipoprotein(a) formation: A randomized clinical trial. J. Am. Med. Assoc. 2023;330(11):1042–1053.
[Crossref] [Google Scholar] [PubMed]
- Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382(3):244–255.
[Crossref] [Google Scholar] [PubMed]
- O’Donoghue ML, Rosenson RS, Gencer B, López JA, Lepor NE, Baum SJ, et al. Small interfering RNA to reduce lipoprotein(a) in cardiovascular disease. N Engl J Med. 2022;387(20):1855–1864.
[Crossref] [Google Scholar] [PubMed]
- Nissen SE, Wolski K, Balog C, Swerdlow DI, Scrimgeour AC, Rambaran C, et al. Single ascending dose study of a short interfering RNA targeting lipoprotein(a) production in individuals with elevated plasma lipoprotein(a) levels. J. Am. Med. Assoc. 2022;327(17):1679–1687.
[Crossref] [Google Scholar] [PubMed]
- Nissen SE, Linnebjerg H, Shen X, Wolski K, Ma X, Lim S, et al. Lepodisiran, an extended-duration short interfering RNA targeting lipoprotein(a): A randomized dose-ascending clinical trial. J. Am. Med. Assoc. 2023;330(21):2075–2083.
[Crossref] [Google Scholar] [PubMed]
Citation: Rendler J, Murphy M, Yeang C (2024) Lipoprotein(a) is a Prevalent yet Vastly Underrecognized Risk Factor for Cardiovascular Disease. Health Care Curr Rev. 12.397.
Copyright: © 2024 Rendler, Murphy and Yeang. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

