Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Short Communication - (2022) Volume 10, Issue 3
Leukocytoclastic Vasculitis and the COVID-19 Pandemic
Andrew B. Dicks* and Bruce H. GrayReceived: 24-Mar-2022, Manuscript No. JVMS-22-15833; Editor assigned: 28-Mar-2022, Pre QC No. JVMS-22-15833(PQ); Reviewed: 11-Apr-2022, QC No. JVMS-22-15833; Revised: 14-Apr-2022, Manuscript No. JVMS-22-15833(R); Published: 26-Apr-2022, DOI: 10.35841/2329-6925.22.10.446
Description
The coronavirus disease 2019 (COVID-19) pandemic, caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has resulted in the infection of hundreds of millions of patients since its discovery in December 2019. With this, there have been a myriad of symptoms associated with the infection. While the infection is most commonly associated with its respiratory effects, extrapulmonary manifestations including numerous dermatologic manifestations are common. Prior reports have demonstrated that approximately 20% of patients with COVID-19 develop a cutaneous manifestation [1]. These cutaneous findings can be quite varied, ranging from urticarial and maculopapular rashes to livedo reticularis and chilblains [2]. Amongst these manifestation, there have been several case reports of Leuko Cytoclasic Vasculitis (LCV) associated with COVID-19 infections [2,3].
LCV commonly affects the skin of the legs, as a small-vessel vasculitis characterized by discrete purpuric lesions. These are caused by deposition of immune complexes in the dermal capillaries and venules and complement-mediated damage with small vessels [4]. Half of all cases of LCV are idiopathic, or primary. Secondary forms of LCV have been associated with ANCA-associated vasculitis; immune complex vasculitis such as cryoglobulinemic vasculitis, IgA-vasculitis, hypocomplementemic urticarial vasculitis, and IgG/IgM vasculitis; vasculitis associated with systemic diseases such as rheumatoid arthritis, systemic lupus erythematosus, and sarcoidosis; and vasculitis secondary to infections, medications, sepsis, or cancer [5]. Numerous medications have been implicated, including several vaccines such as the influenza, Hepatitis B (HBV), Bacille Calmette-Guerin (BCG), and Human Papilloma Virus (HPV) vaccines [6]. The typical clinical manifestation of LCV consists of symmetrically distributed, non-blanchable purpuric papules, mostly located on the lower legs or dependent areas (Figures 1A and 1B) [7]. Patients with LCV are often asymptomatic; however, those with symptoms often describe burning, itching, or pain of the involved skin [5]. The presence of fever, weight loss or other constitutional symptoms is often suggestive of a systemic vasculitis. These clinical signs of LCV are routinely confirmed with skin biopsy. Most cases of cutaneous LCV are mild and resolve with supportive measures; if LCV is more chronic or resistant, oral corticosteroids can be used. For medication induced LCV, withdrawal of the drug is crucial for resolution. When LCV is associated with systemic illness, other immunosuppressants may be necessary beyond corticosteroids.
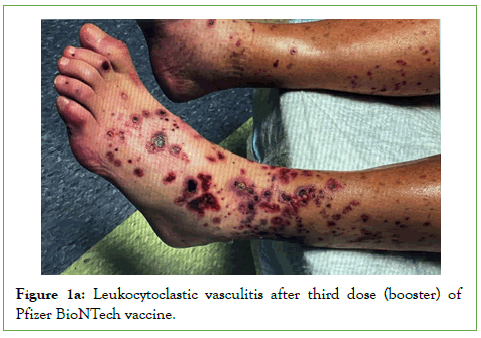
Figure 1a: Leukocytoclastic vasculitis after third dose (booster) of Pfizer BioNTech vaccine.
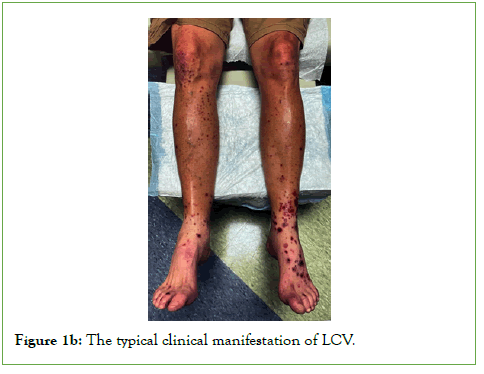
Figure 1b: The typical clinical manifestation of LCV.
While it is unclear as to the exact etiology of why COVID-19 infection causes LCV, it is theorized that the Angiotensin-Converting Enzyme 2 (ACE2) receptor for which the SARS-CoV-2 virus uses to enter cells is also expressed on skin cells [8]. Interestingly, while LCV has been seen in symptomatic patients with the COVID-19 infection, there are more published case reports of LCV occurring after the SARS-CoV-2 vaccine [6,7,9-12]. Besides LCV, there have been numerous reports of cutaneous manifestations after receiving the SARS-CoV-2 vaccine, many of which are similar to those associated with the COVID-19 infection. The most commonly reported cutaneous manifestations are Vaccine-Related Eruption of Papules and Plaques (V-REPP), bullous pemphigoid-like, dermal hypersensitivity reactions, herpes zoster, lichen planus-like, and pernio [9].
The mechanism of SARS-CoV-2 vaccine induced LCV is unclear but could potentially be driven by off target activation of B/T cells, antibody formation, and immune complex deposition within small vessels [7]. Pathology from cases has been varied when available, with cases reporting evidence of IgA deposition, IgM deposition, IgA and IgM deposition, fibrinogen deposition, and IgA/IgM/C3 deposition [7,11,13-17]. The Pfizer BioNTech, Oxford/AstraZeneca ChAdOx1-S, Moderna, Sinovac-Coronovac, and Bharat Biotech COVAXIN vaccines have all been described as resulting in the development of LCV [7,10,15,16-23]. There are cases reporting onset of LCV after the first, second, and third (booster) dose of the various vaccines [7,10,12]. Onset of symptoms typically occurs with 7-10 days after vaccine injection. The majority of cases involved cutaneous LCV with only one case describing systemic issues; in this case, the patient developed acute interstitial nephritis despite use of systemic corticosteroids [19]. Management centers on corticosteroid therapy, either intravenous, oral, or topical, with few reports describing use of antihistamines or antibiotics [12,16,17]. Outcomes were typically favorable with LCV being self-limited with partial or full resolution with the above treatment measures. With regards to LCV recurrence, one case described development of LCV after the first dose of Pfizer BioNTech which was treated with oral corticosteroids with subsequent recurrence of LCV after the second vaccine dose [6].
Conclusion
While remaining relatively rare, LCV has been shown to be a cutaneous manifestation of both the COVID-19 infection as well as a reaction related to the SARS-CoV-2 vaccine. In these cases, LCV is typically localized to the skin without systemic manifestations and is effectively treated with corticosteroids with both topical and systemic therapy. Additional studies are necessary to further understand the link between LCV and the SARS-CoV-2 virus and vaccine and to establish optimal management strategies.
REFERENCES
- Recalcati S. Cutaneous manifestations in COVID-19: A first perspective. J Eur Acad Dermatol Venereol. 2020; 34(5):e212-e213.
[Crossref] [Google scholar] [PubMed]
- Genovese G, Moltrasio C, Berti E, Marzano AV. Skin manifestations associated with COVID-19: Current knowledge and future perspectives. Dermatology. 2021; 237(1):1-12.
[Crossref] [Google scholar] [PubMed]
- Wong K, Farooq Alam Shah MU, Khurshid M, Ullah I, Tahir MJ, Yousaf Z. COVID-19 associated vasculitis: A systematic review of case reports and case series. Ann Med Surg. 2022; 74:103-249.
[Crossref] [Google scholar] [Pub Med]
- Baigrie D, Bansal P, Goyal A, Crane JS. Leukocytoclastic Vasculitis. In: StatPearls. StatPearls Publishing. 2021.
[Crossref] [Google scholar] [PubMed]
- Fraticelli P, Benfaremo D, Gabrielli A. Diagnosis and management of leukocytoclastic vasculitis. Intern Emerg Med. 2021; 16(4):831-841.
[Crossref] [Google scholar] [PubMed]
- Cohen SR, Prussick L, Kahn JS, Gao DX, Radfar A, Rosmarin D. Leukocytoclastic vasculitis flare following the COVID-19 vaccine. Int J Dermatol. 2021; 60(8):1032-1033.
[Crossref] [Google scholar] [PubMed]
- Fiorillo G, Pancetti S, Cortese A. Leukocytoclastic vasculitis (cutaneous small-vessel vasculitis) after COVID-19 vaccination. J Autoimmun. 2022; 127:102-783.
[Crossref] [Google scholar] [PubMed]
- Iraji F, Galehdari H, Siadat AH, Bokaei Jazi S. Cutaneous leukocytoclastic vasculitis secondary to COVID-19 infection: A case report. Clin Case Rep. 2021; 9(2):830-834.
[Crossref] [Google scholar] [PubMed]
- McMahon DE, Kovarik CL, Damsky W. Clinical and pathologic correlation of cutaneous covid-19 vaccine reactions including v-repp: a registry based study. J Am Acad Dermatol. 2021; 190-962.
[Crossref] [Google scholar] [PubMed]
- Dicks AB, Gray BH. Images in vascular medicine: Leukocytoclastic vasculitis after covid-19 vaccine booster. Vasc Med. 2022; 27(1):100-101.
[Cross ref] [Google scholar] [Pub Med]
- Bostan E, Gulseren D, Gokoz O. New-onset leukocytoclastic vasculitis after COVID-19 vaccine. Int J Dermatol. 2021.
[Cross ref] [Google scholar] [Pub Med]
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022; 35(3):e15279.
[Cross ref] [Google scholar] [Pub Med]
- Fritzen M, Funchal GDG, Luiz MO, Durigon GS. Leukocytoclastic vasculitis after exposure to COVID-19 vaccine. An Bras Dermatol. 2022; 97(1):118-121.
[Cross ref] [Google scholar] [Pub Med]
- Carrillo-Garcia P, Sánchez-Osorio L, Gómez-Pavón J. Leukocytoclastic vasculitis in possible relation to the BNT162b2 mRNA COVID-19 vaccine. J Am Geriatr Soc. 2022.
[Cross ref] [Google scholar] [Pub Med]
- Bencharattanaphakhi R, Rerknimitr P. Sinovac COVID-19 vaccine-induced cutaneous leukocytoclastic vasculitis. JAAD Case Rep. 2021; 18:1-3.
[Cross ref] [Google scholar] [Pub Med]
- Kar BR, Singh BS, Mohapatra L, Agrawal I. Cutaneous small-vessel vasculitis following COVID-19 vaccine. J Cosmet Dermatol. 2021; 20(11):3382-3383.
[Cross ref] [Google scholar] [Pub Med]
- Larson V, Seidenberg R, Caplan A, Brinster NK, Meehan SA, Kim RH. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J Cutan Pathol. 2022; 49(1):34-41.
[Cross ref] [Google scholar] [Pub Med]
- Sandhu S, Bhatnagar A, Kumar H, et al. Leukocytoclastic vasculitis as a cutaneous manifestation of ChAdOx1 nCoV-19 corona virus vaccine (recombinant). Dermatol Ther. 2021; 34(6):e15141.
[Cross ref] [Google scholar] [Pub Med]
- Missoum S, Lahmar M, Khellaf G. Leukocytoclastic vasculitis and acute renal failure following inactivated SARS-CoV-2 vaccine. Nephrol Ther. 2021; S1769-7255.
[Cross ref] [Google scholar] [Pub Med]
- Jin WJ, Ahn SW, Jang SH, Hong SM, Seol JE, Kim H. Leukocytoclastic vasculitis after coronavirus disease 2019 vaccination. J Dermatol. 2022; 49(1):e34-e35.
[Cross ref] [Google scholar] [Pub Med]
- Erler A, Fiedler J, Koch A, Schütz A, Heldmann F. A case of leukocytoclastic vasculitis after vaccination with a SARS-CoV2-vaccine - a case report. Arthritis Rheumatol. 2021.
[Cross ref] [Google scholar] [Pub Med]
- Shahrigharahkoshan S, Gagnon LP, Mathieu S. Cutaneous leukocytoclastic vasculitis induction following chadox1 ncov-19 vaccine. Cureus. 2021; 13(10):e19005.
[Cross ref] [Google scholar] [Pub Med]
- Ireifej B, Weingarten M, Dhamrah U, Weingarten M, Hadi S. Leukocytoclastic Vasculitic Rash Following Second Dose Of Moderna COVID-19 Vaccine. J Investig Med High Impact Case Rep. 2022; 10:23247096211066284.
[Cross ref] [Google scholar] [Pub Med]
Citation: Dicks AB, Gray BH (2022) Leukocytoclastic Vasculitis and the COVID-19 Pandemic. J Vasc Surg. 10:446.
Copyright: © 2022 Dicks AB, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

