Indexed In
- Open J Gate
- Genamics JournalSeek
- ResearchBible
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 10, Issue 9
Knowledge and Perception Assessment of Pharmacy Technicians on Biosimilars at the University Hospital Center-Tlemcen
Amina Berradia*, Chebaiki nebia and Metahri AbderrahimReceived: 15-Aug-2021 Published: 23-Sep-2021, DOI: 10.35248/2167-7956.21.10.179
Abstract
Biosimilars are similar products, but not identical, to a reference biological drug already approved by the authorities, whose protection certificate has expired. Biosimilars have recently been introduced at Tlemcen University Hospital Center (TUHC). Pharmacy technicians are essential health actors for good distribution and use of bio-drugs in particular biosimilars within the TUHC. For this, we have done a study to assess the knowledge of pharmacy technicians on biosimilars as well as their opinion on their efficacy-safety profiles. The data collection was carried out by the distribution of a questionnaire with 12 questions. We have analyzed the questionnaires of the 10 pharmacy technicians who agreed to join our study.
More than half of pharmacy technicians could not correctly define a biosimilar. In addition, according to the answers provided, the stages of develo in order to raise aw pment and approval of biosimilars are not well known. Continuous training on the topic of biosimilars is necessary areness in this category of the importance of rigorous monitoring of these treatments within the TUHC.
Regarding the accessory goal of the questionnaire attributed to pharmacy technicians, to provide their opinions on the efficacy and safety of biosimilars according to their daily professional experience, a large part of pharmacy technicians has expressed a satisfaction with the efficiency and safety of these drugs. However, since a certain category of pharmacy technicians affirms to witness suspicion of side effects of biosimilar treatment without informing the Central Pharmacy, it is imperative to raise awareness of the need to declare them to regional and national pharmacovigilance centers.
Keywords
Biosimilars; Pharmacy technicians; Biological drugs; Pharmacovigilance
Introduction
Biological medicinal products have revolutionized the management and fate of many diseases in terms of morbidity/mortality and quality of life [1-3]. Biosimilars are similar products, but not identical, to a reference biological drug already approved by the authorities, whose protection certificate has expired. Biosimilars, by their intrinsic nature, the complexity of their production, their quality controls and their regulatory assessment, cannot be considered as generics of biological drugs [4]. During this last decade, several biosimilar molecules have had access to the Algerian market and are used in different health structures. Their commercialization requires rigorous monitoring of their effectiveness and side effects. Biosimilars have recently been introduced at Tlemcen University Hospital Center (TUHC). Their use has become preponderant within this establishment. Pharmacy technicians are essential health actors for good distribution and use of biodrugs in particular biosimilars within the TUHC. For this, we have done a study to assess the knowledge of this category on biosimilars as well as their opinion on their efficacy-safety profiles.
Materials and Methods
This is a descriptive observational study; it has been provided with a questionnaire for the pharmacy technicians of TUHC. The choice of the TUHC as a study environment was motivated by the high workforce of pharmacy technicians compared to other health structures.
The consent of pharmacy technicians was implied by filling or not questionnaires.
The data collection was carried out by the distribution of a questionnaire with 12 questions (Figure 1), aiming to assess the state of knowledge of pharmacy technicians on biosimilars, as well as their opinion on their efficacy-safety profiles. The data were collected from December 20, 2019 to January 20, 2020. Data entry was performed by the SPSS Statistics 25 software. The results analysis was performed with the same software. The results are expressed as a percentage for qualitative variables.

Figure 1: Questionnaire sheet assigned to pharmacy technicians.
Results
During the period from December 20, 2019 to January 20, 2020, we interviewed 10 pharmacy technicians (Figure 2) shows their distribution according to their belonging service. 20% of pharmacy technicians asked belong to the medico-surgical emergencies department of the (TUHC).
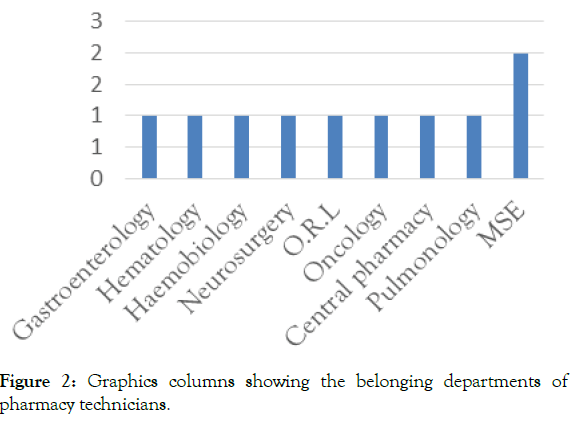
Figure 2: Graphics columns showing the belonging departments of pharmacy technicians.
Presentation of questionnaire-results
General information on biological drugs: Unfortunately, only 20% of the questioned pharmacy technicians provided a correct answer to this question. 30% of them provided only a partial response, in other words, they checked partially the correct question-proposals. Almost one-third of them gave a false response and 20% of them did not provide an answer (Figure 3).
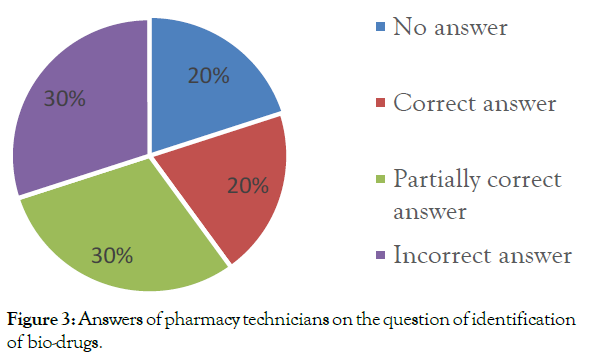
Figure 3: Answers of pharmacy technicians on the question of identification of bio-drugs.
As for defining a biosimilar, the percentage of pharmacy technicians that have provided a correct response was below average (40%) (Figure 4).
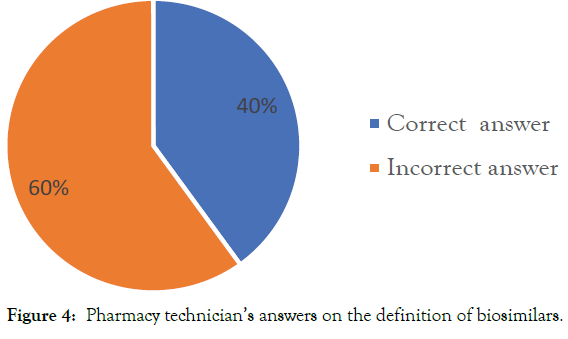
Figure 4: Pharmacy technician’s answers on the definition of biosimilars.
Information on the approval process for a biosimilar: The majority of pharmacy technicians (80%) provided only partially correct answers. 20% of them did not provide a response (Figure 5).
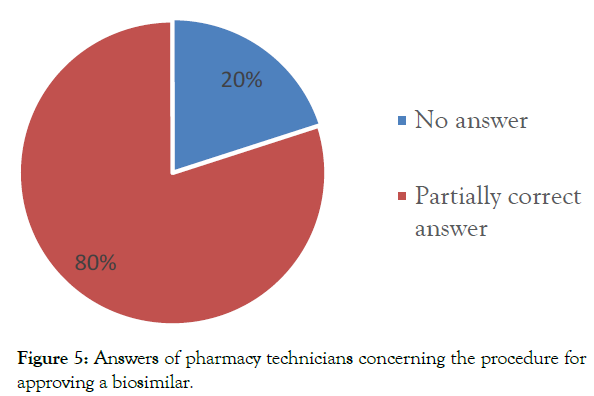
Figure 5: Answers of pharmacy technicians concerning the procedure for approving a biosimilar.
Perception of the efficacy of biosimilars: Half of the questioned pharmacy technicians (50%) gave a negative response and therefore think that biosimilar would not have an effectiveness equivalent to its reference product (Figure 6).
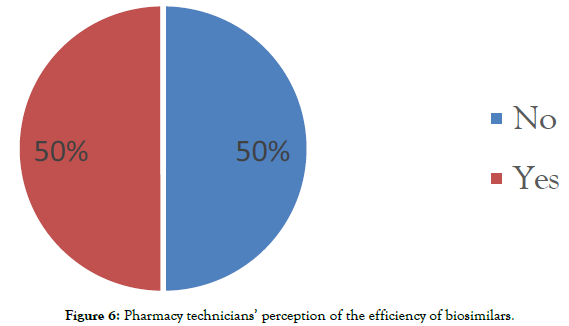
Figure 6: Pharmacy technicians’ perception of the efficiency of biosimilars.
Perception of the safety of biosimilars: Half of the questioned pharmacy technicians (50%) think that the use of biosimilar would not be as safe as that of the reference drug (Figure 7).
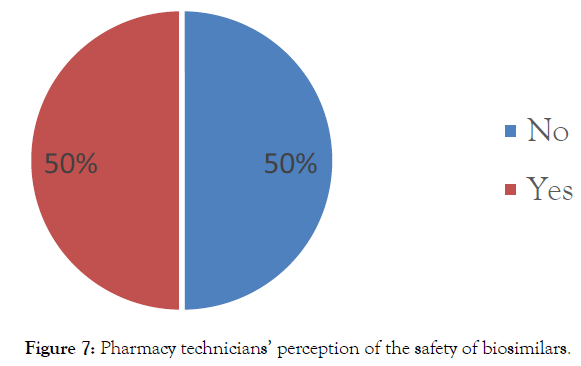
Figure 7: Pharmacy technicians’ perception of the safety of biosimilars.
Information on the substitution of biosimilar by pharmacists: 50% of pharmacy technicians interviewed believe that the pharmacist is not allowed to substitute between the biosimilar and its reference product (Figure 8).
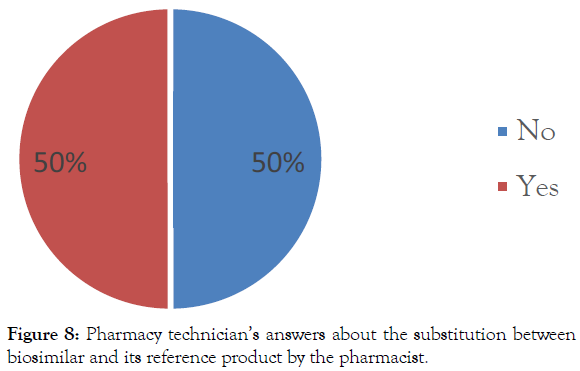
Figure 8: Pharmacy technician’s answers about the substitution between biosimilar and its reference product by the pharmacist.
Information on the economic advantage of biosimilars: Most pharmacy technicians (70%) provided an approved response and think, thus, that biosimilars have an economic advantage and allows easy access to treatment (Figure 9).
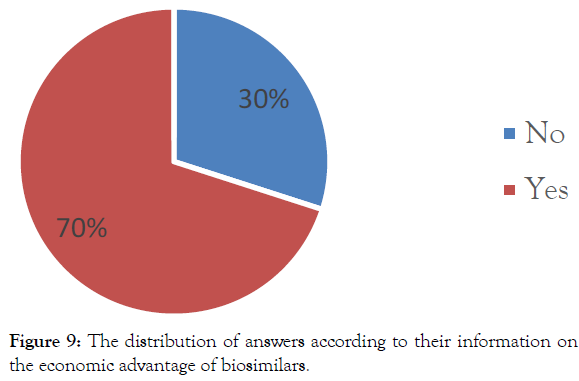
Figure 9: The distribution of answers according to their information on the economic advantage of biosimilars.
Survey of their experience with biosimilars at TUHC: When pharmacy technicians were interviewed on how biological drugs are prescribed within the TUHC, The proposal "INN: International Non-proprietary Name” was the most checked (60%) (Figure 10).
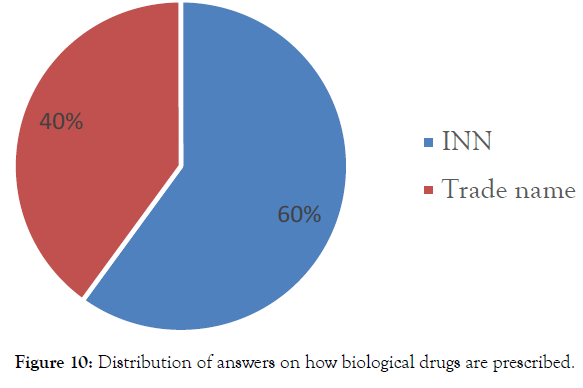
Figure 10: Distribution of answers on how biological drugs are prescribed.
In addition, the vast majority of pharmacy technicians (88%) report that biosimilar are the same efficacy as the reference products. According to them, it has not been reported differences in efficacy between the 2 bio-drugs (Figure 11).
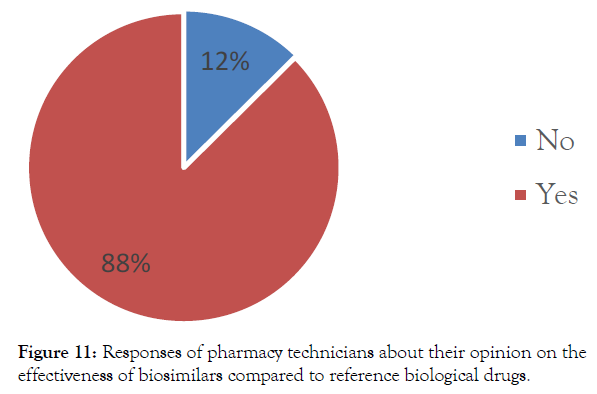
Figure 11: Responses of pharmacy technicians about their opinion on the effectiveness of biosimilars compared to reference biological drugs.
Nevertheless, almost half of the pharmacy technicians interviewed (40%) report that patients under biosimilar complain more often from side effects than those treated with reference bio drugs (Figure 12).
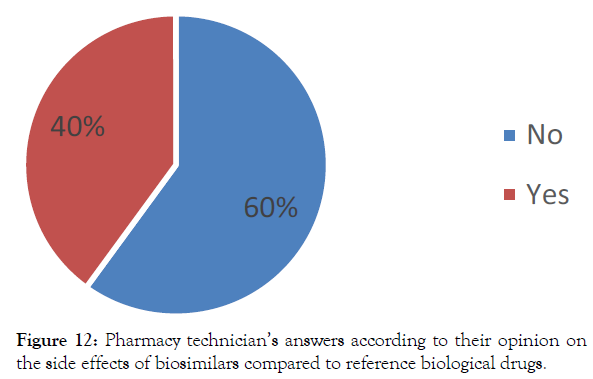
Figure 12: Pharmacy technician’s answers according to their opinion on
the side effects of biosimilars compared to reference biological drugs.
All pharmacy technicians have affirmed that they do not report side effects to central hospital pharmacy or to pharmacovigilance centers (Figure 13).
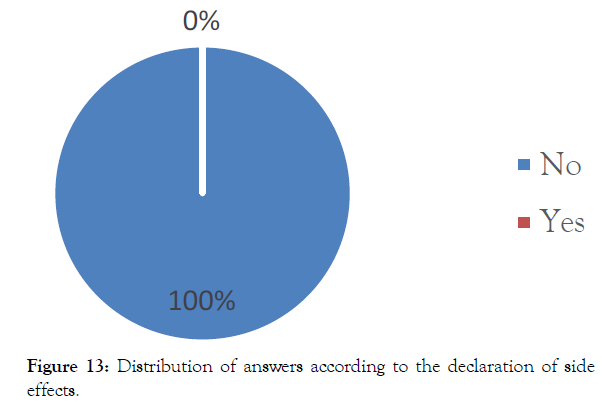
Figure 13: Distribution of answers according to the declaration of side effects.
Discussion
During this last decade, several biosimilar molecules have had access to the Algerian market and are used in different health structures. For this, we decided to study them at the level of the TUHC given their recent and preponderant introduction within this establishment. 06 biosimilars were used at the TUHC during the period of our study: EPOTIN®, AVONEX®, JUSLINE N®, INSUDAL BASAL®, CANMAB®, REMSIMA®, The REMSIMA® is the biosimilar that has been most recently introduced to the TUHC. All these biosimilars are imported. After distribution of the questionnaire among pharmacy technicians, we have collected 10 questionnaires. In other words, we analyzed the questionnaires of the 10 pharmacy technicians who agreed to join our study.
Several Press articles [5-7] report that some pharmaceutical firms on the Algerian territory consider the beginning of biosimilar development in Algeria, to limit the import cost and to meet the needs of the market. The questionnaire attributed to pharmacy technicians was intended to assess their state of knowledge on biosimilars, as well as their opinion on their efficacy-safety profiles. The questionnaire attributed to pharmacy technicians was intended to assess their state of knowledge on biosimilars, as well as their opinion on their efficacy-safety profiles.
According to the responses of pharmacy preparators on the questionnaire that most pharmacy technicians have insufficient information on bio-drugs and biosimilars. In the first question, only 20% of pharmacy technicians could identify the drugs that are: Remicade® and Prolia®, also more than half of them could not correctly define a biosimilar; the word "generic" was cited in several responses. A biosimilar is always mistaken for a generic. In addition, according to the answers provided, the stages of development and approval of biosimilars are not well known.
Continuous training on the topic of biosimilars is necessary in order to raise awareness in this category of the importance of rigorous monitoring of these treatments within the TUHC. Also, 50% of the pharmacy technicians interviewed believe that the pharmacist has the authorization to substitute a reference bio drug by its biosimilar, but the Algerian regulation, as we have already specified, is not clear in this regard. It is also found that the majority of pharmacy technicians know that biosimilar is easy to access and has an economic advantage.
Regarding the accessory goal of the questionnaire attributed to pharmacy technicians, to provide their opinions on the efficacy and safety of biosimilars according to their daily professional experience, a large part of pharmacy technicians has expressed a satisfaction with the efficiency and safety of these drugs. However, since a certain category of pharmacy technicians affirms to witness suspicion of side effects of biosimilar treatment without informing the Central Pharmacy, it is imperative to raise awareness of the need to declare them to regional and national pharmacovigilance centers. Faced with insufficient information from pharmacy technicians, important health players within the TUHC, we designed a leaflet summarizing the main characteristics of biosimilars and we distributed it to all the pharmacy technicians who participated in the study.
Conclusion
In recent years, many patents of biological drugs have expired, which has led to the emergence of biosimilars. Some of these drugs are used at the TUHC. These medications reduction in the period of drug stock-outs and have an economic advantage, but many health professionals at the TUHC have insufficient knowledge of these drugs, their differences with generic drugs and how to monitor them. In the end, we strongly hope that our study has helped to better identify the situation of the biosimilar, meet certain limits in their way of being used and specially to test and enrich the knowledge of a very large category of health actors who are pharmacy technicians. This will undoubtedly have a favorable impact on the management of patients under biological drugs at the level of TUHC.
REFERENCES
- Pinheiro D. Bilan en 2012 sur l’utilisation des bio similaires: Un équivalent du bio médicament de référence ou un générique biologique? Université de Lorraine. 2012.
- Gregory F, Gabay C. Effets secondaires des traitements biologiques. Rev Med CH. 2017;13:542-548.
- ANSM: Les médicaments bio similaires-ANSM: Agence Nationale de Sécurité du Médicament et des produits de santé cite. 2020.
- Argentin A. Laura. Production, quality control and regulation of biosimilar drugs: A challenge for the pharmaceutical industry. Doctor of pharmacy. Toulouse III University, Paul Sabatier. 2014.
- Bellagha H. Health/Medicine: The biosimilars drug (almost) natural, Reporters. 2019.
- Benhalima B. Directives and regulations are essential, Elwatan, June 16, 2019.
- Santénews. Biosimilars will allow Algeria to reduce its import bill by up to minus $350 million. 2019.
Citation: Berradia A, nebia C, Abderrahim M (2021) Knowledge and Perception Assessment of Pharmacy Technicians on Biosimilars at the University Hospital Center-Tlemcen. J Biol Res Ther 10:179.
Copyright: © 2021 Berradia A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

