Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2020) Volume 11, Issue 6
Is a Real Time-Polymerase chain Reaction a Reliable Confirmatory Test for COVID-19?
Abdelmonem M Ali1, Alfatih Aboalbasher Yousif2, Ahmed O Abuelhassan1, Ebtehal M fawzi1, Sheima A Elbasheer3 and Nagia S Ahmed42Star Care Medical Laboratory, Sudan
3Department of Microbiology, College of Medical Laboratory Science, University of Alzaiem Al Azhari, Sudan
4Department of Clinical Chemistry, College of Medical Laboratory Science, Al Gezeria University, Sudan
Received: 19-Sep-2020 Published: 27-Oct-2020, DOI: 10.35248/2155-9864.20.11.442
Abstract
Importance: Corona virus Diseases 2019 (COVID-19) is an unprecedented global health pandemic that occurs in the twentieth century, caused by a virus genra called SARS-CoV-2 which infects the lower respiratory system. Started in Wuhan city in Chine, then disease has spread worldwide until the WHO declared as pandemic disease. Recent View: The current pandemic SARS-CoV-2. Confuses the scientific and the medical community about which accurate diagnostic tool should be relied on to diagnosis COVID-19. RT-PCR is considered as a gold stander technique for COVID-19, however, there are a few viewpoints doubting underestimating its sensitivity and specificity. Here in the current narrative review, we have shed light on the both opinions and we have added our perspectives, as well. Critical concept: In spite of a little concern about the reliability of RT-PCR, up to date RT-PCR has been accredited as a fundamental diagnostic technique. Nevertheless, to eliminate this concern regarding the sensitivity and specificity of RT-PCR; there are efforts from scientists to develop more molecular diagnostic technique; such as multiplex PCR. Future prospect: To reduce the variation on the RT-PCR result report, it would be better; if there is a universal guideline or protocol to be applied in this critical situation.
Keywords
COVID-19; Real Time-PCR; SARS-CoV-2; Sensitivity; Specificity
Introduction
Since December 2019, Corona Virus which is later known as COVID-19 has occupied top news all around the world. In the beginning, Corona virus started in Wuhan city in China as local outbreak. But later the disease has spread out all around the world until the WHO declared COVID-19 as a pandemic disease. It has not only represented a critical issue for health authorities, but also caused concerns for political leaders, because of its direct impact on economic situation. Whereas, most of the businesses over the world are lockdown [1]. This is an unprecedented global health pandemic at least in the twentieth century. COVID-19 is one of the viruses that infects lower respiratory system and causes severe acute respiratory syndrome (SARS). The virus which caused COVID-19 is called SARS-CoV2 [2,3]. In the beginning, the COVID-19 genome was unknown, it has been sequenced and became available online on the first of January 2020 [4]. Then after knowing the SARS-COV2 genome sequence, another controversial issue has started in the medical community, regarding which analytical tool could be used as a reliable mechanism for diagnosis ofCOVID-19. Actually, up to date, the diagnosis of COVID-19 is basically based on RT-PCR, in addition to CT, and recently serological antibody detection is also used. However, from all these techniques, RTPCR is considered as a gold stander for diagnosis of COVID-19 as established in the previous reports. Up to date the RT-PCR is accredited globally as laboratory technique for identification of SARS-CoV2 [5]. Nevertheless, there are some opposing views around the sensitivity of RT-PCR in the detection of SARSCoV- 2.
Here, this narrative review has focused on a degree of reliability of RT-PCR and attempted to evaluate and clarify the literature regarding the accuracy of RT-PCR and makes recommendations based on this literature.
Molecular diagnosis of COVID-19
RT–PCR is a molecular diagnostic technique used to detect presence of specific genetic material in any pathogen, including a virus. The principle of this technique based on conversion of RNA to a complementary DNA (cDNA) by a process called reverse transcription. Then, the PCR amplifies the cDNA exponentially DNA which is detected by real- time using fluorescence probes [6].
At the current pandemic situation, RT-PCR is used to transcribe and amplify the specific sequence(s) of SARS-CoV-2 from target specimen (nasal or nasopharyngeal swabs) [7].
Pros of RT-PCR
Some of the published data showed that the sensitivity of the RT-PCR reached up to 95% for diagnosis of COVID-19. That means enabling early detection of low viral load [8]. In spite of that, there are different laboratory methods to detect viruses such as Cell Culture, Immunofluorescence (IF) Assay, Immunoblotting, Flow Cytometry (FCM) and Transmission Electron Microscopy (TEM), but among them, the RT-PCR is preferred to be used for detection of COVID-19, because it may be able to provide results within hours or in maximum within 2 days [9-11]. The cost of RTPCR detection test is cheaper if compared with other molecular diagnostic techniques [12].
Cons of RT-PCR
There are some perspectives that should be taken into consideration around the sensitivity of the RT-PCR test in the detection of COVID-19. Firstly, the similarity of genome between SARS-CoV-2 and SARS-CoV, whereas, about 82% of nucleotide identity with some of the primer-probes of SARS-CoV and other SARS-related viruses can lead to have a cross-reaction [13,14]. Secondly, critical concern of low sensitivity as reported for RT-PCR assays, especially when patients are in the early stages [15,16]. Thirdly, the RTPCR results revealed a fluctuating trend. Theses may happen by inadequate viral material in the specimen, laboratory error during sampling collection or transportation [17].
In addition to that, the RT-PCR machine requires tremendous knowledge for its technical operation, whereas the operator who works on the machine should be well trained [18]. RT–PCR could not be used to detect past infections (which may help to understand the development and spread of the virus) [19]. So, to reduce the potential risk of false-negative PCR, some studies have recommended to examine chest CT for clinically suspected patients with negative initial RT-PCR [20].
Discussion
False-negative and false-positive results of the RT-PCR still are considered as one of the critical issues in the diagnosis of COVID-19. Especially, when patients with a negative result, couldn’t exclude the possibility of COVID-19 infection, and should not take the only RT-PCR result as a criterion for patient management decisions [21]. As recommended by experts, it’s important to run another confirmatory test such as a CT or antibodies test to emphasize the RT-PCR result [22].
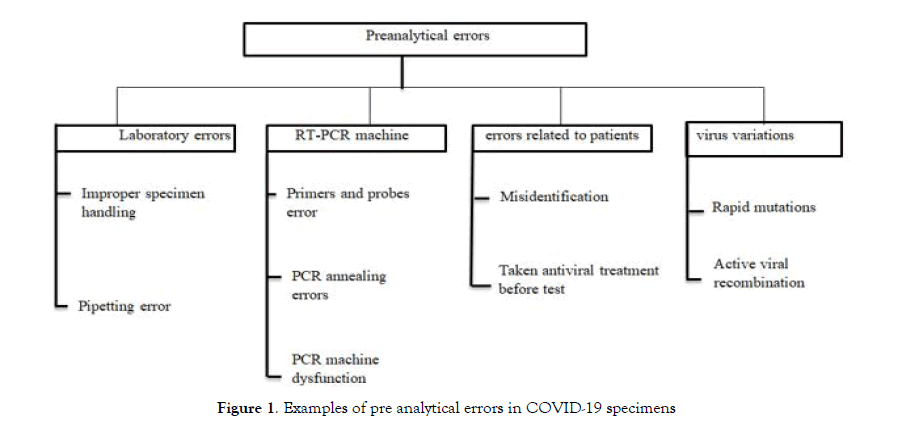
Pre analytical errors
Due to pre analytical errors, perhaps miss-diagnosis occurs in patient infected by COVID-19. These errors could be divided into many categories [23] see Figure 1.
Modifications of RT-PCR
One of the significant drawbacks of the diagnosis of severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) is sensitivity. To minimize the risk of false-negative results, there was a need to develop a convenient RT-PCR to more advanced RT- PCR, therefore, appears a multiplex rRT-PCR. Whereas, in multiplex PCR, occurs an amplification of more than one target gene which leads to the prevention of amplification of less abundant targets genes and subsequently increases the sensitive detection of target genes. This mechanism confirms the WHO recommendation regarding detecting at least two different targets gene on the COVID-19 virus genome [24,25].Another modification of molecular diagnostic of COVID-19 is COVID-19-nsp2, whereas did not amplify other human-pathogenic corona viruses and respiratory viruses that means only amplify the Coronavirus-2 target genes, to some extent it could be highly sensitive and specific [26].
Figure 1: Examples of pre analytical errors in COVID-19 specimens
Author's Prespective
According to the published data at this point, the degree of reliability of RT-PCR specificity and sensitivity for the diagnosis of COVID-19 is extremely acceptable, especially after the modifications which have been entered by using multiplex rRT-PCR.
Nevertheless, there is a little concern about the biological validity of quantitative data of RT-PCR results. In our perspective view, we think that, if a global agreement to determine the quality is implemented, and quantity of RNA control, and guidelines about basic issues such as how to write unified result report for COVID-19 all over the world, all these amendments may be helpful to reduce the variations between RT-PCR results. Finally, the authors of this review support and recommended that, as long as RT-PCR is considered as gold stander and reliable technique for diagnosis of COVID-19, it would be better if the future efforts focus on resolving the limited negative drawback of RT-PCR (as mentioned above). They could benefit from misconception which accompanied the current pandemicCOVID-19 result (either due to false negative or false positive result), and through correction of this misconception by scientific explanation, they could reach to a satisfactory result. Moreover, by figuring out the accurate diagnostic tool for the current pandemic COVID-19, the world will be ready if the second wave of SARS-CoV-2 is to come.
Conclusion
To sum up, a rapid changing of new research findings represents major Challenges to laboratory diagnosis of the unprecedented PandemicCOVID-19. However, RT-PCR assay is still a fundamental method to be applied for the detection of SARS-CoV-2 to date.
Acknowledgement
Our great Thanks are to Mr: Yassir Aboalbasher Yousif and the Sudanese Medical Laboratory Technologists in Oman (SMLT) for their logistic support.
Author Contributions
Abdelmonem M Ali, Ahmed O Abuelhassan, Ebtehal M fawzi, Nagia S Ahmed, Shiema A Elbasheer have contributed equally in conceptualizing and writing the original draft; Alfatih Abo Albasher Yousif has edited and reviewed it the final draft.
Conflict of Interest
The authors declare that they have no conflict of interest.
REFERENCES
- Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382:872-874.
- Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int Surg J. 2020;76:p.71-76
- Peñarrubia L, Ruiz M, Porco R, Rao SN, Juanola-Falgarona M, Manissero D, et al. Multiple assays in a real-time RT-PCR SARS-CoV-2 panel can mitigate the risk of loss of sensitivity by new genomic variants during the COVID-19 outbreak. Int J Infect Dis. 2020;97:225-229.
- Lu X, Wang L, Sakthivel SK, Whitaker B, Murray J, Kamili S, et al. US CDC real-time reverse transcription PCR panel for detection of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26:1654.
- Shen M, Zhou Y, Ye J, Al-Maskri AA, Kang Y, Zeng S, et al. Recent advances and perspectives of nucleic acid detection for coronavirus. J Pharm Anal. 2020;10:97-101.
- Feng W, Newbigging AM, Le C, Pang B, Peng H, Cao Y, et al. Molecular diagnosis of COVID-19: challenges and research needs. Anal Chem. 2020;92:10196-10209.
- Afzal A. Molecular diagnostic technologies for COVID-19: Limitations and challenges. J Adv Res. 2020.
- D'Cruz RJ, Currier AW, Sampson VB. Laboratory testing methods for novel severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). Front Cell Dev Biol. 2020;8.
- Kumar P. Methods for rapid virus identification and quantification. Mater Adv. 2013;3:207.
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265-269.
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020; 382:929-936.
- Ji T, Liu Z, Wang G, Guo X, Lai C, Chen H, et al. Detection of COVID-19: A review of the current literature and future perspectives. Biosens Bioelectron. 2020;166:112455.
- Katz AP, Civantos FJ, Sargi Z, Leibowitz JM, Nicolli EA, Weed D, et al. False‐positive reverse transcriptase polymerase chain reaction screening for SARS‐CoV‐2 in the setting of urgent head and neck surgery and otolaryngologic emergencies during the pandemic: Clinical implications. Head & Neck. 2020;42:p.1621- 1628.
- US Food and Drug Administration. Accelerated emergency use authorization (EUA) summary COVID-19 RT-PCR test (Laboratory Corporation of America).
- Ortiz-Prado E, Simbaña-Rivera K, Gómez-Barreno L, Rubio-Neira M, Guaman LP, Kyriakidis NC, et al. Clinical, molecular and epidemiological characterization of the SARS-CoV2 virus and the Coronavirus disease 2019 (COVID-19), a comprehensive literature review. Diagn Microbiol Infect Dis. 2020;30:115094.
- Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiol. 2020;19:200432.
- Li Y, Yao L, Li J, Chen L, Song Y, Cai Z, et al. Stability issues of RT‐PCR testing of SARS‐CoV‐2 for hospitalized patients clinically diagnosed with COVID‐19. J Med Virol. 2020; 92:p.903-908.
- Osman J, Lambert J, Templé M, Devaux F, Favre R, Flaujac C, et al. Rapid screening of COVID‐19 patients using white blood cell scattergrams, a study on 381 patients. Br J Haematol. 2020;190:718-22.
- Jawerth N. How is the COVID-19 Virus Detected using Real Time RT-PCR. IAEA Bulletin. 2020; 8-11.
- He JL, Luo L, Luo ZD, Lyu JX, Ng MY, Shen XP, et al. Diagnostic performance between CT and initial real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China. Respir Med. 2020;21:105980.
- Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn, 2020;20:p.453-454.
- Kim H, Hong H, Yoon SH. Diagnostic performance of CT and reverse transcriptase-polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiol. 2020;17:201343.
- Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clin Chem Lab Med. 2020;1.
- Ishige T, Murata S, Taniguchi T, Miyabe A, Kitamura K, Kawasaki K, et al. Highly sensitive detection of SARS-CoV-2 RNA by multiplex rRT-PCR for molecular diagnosis of COVID-19 by clinical laboratories. Clin Chim Acta. 2020; 507: p.139-142.
- LeBlanc JJ, Gubbay JB, Li Y, Needle R, Arneson SR, Marcino D, et al. Real-time PCR-based SARS-CoV-2 detection in Canadian laboratories. J Clin Virol. 2020;13:104433.
- Yip CC, Ho CC, Chan JF, To KK, Chan HS, Wong SC, et al. Development of a novel, genome subtraction-derived, SARS-CoV-2-specific COVID-19-nsp2 real-time RT-PCR assay and its evaluation using clinical specimens. Int J Mol. 2020;21:2574.
Citation: Ali AM, Yousif AA, Abuelhassan AO, fawzi EM, Elbasheer SA, Ahmed NS (2020) Is a Real Time-Polymerase chain Reaction a Reliable Confirmatory Test for COVID-19? J Blood DisordTransfus 11: 442. DOI: 10.35248/2155-9864.20.11.442
Copyright: © 2020 Ali AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited