Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 3
Involvement of Pyoverdin, Proteolytic and Lipolytic Enzymes in Pseudomonas aeruginosa Biofilm Formation
Yao Paul Attien1,2*, Comoé Koffi Donatien Benié2,3,4*, Haziz Orou Sina5, Wako-Tianwa Alice Tuo6, Arthur Zebre1, Clément Kouassi Kouassi1, Ibrahim Konaté1, Lamine Baba Moussa5, Adjehi Dadie2 and Mireille Dosso42Department of Food Science and Technology, Laboratory of Biotechnology and Food Microbiology (LMBM), University of Nangui-Abrogoua, Abidjan, Cote D'Ivoire
3Department of Biosciences, Laboratory of Biotechnology, Agriculture and Valorization of Biological Resources, University of Félix Houphouët Boigny, Abidjan, Cote D'Ivoire
4Department of Bacteriology and Virology, Institut Pasteur of Côte d'Ivoire (IPCI), Abidjan, Cote D'Ivoire
5Department of Science and Technology, Laboratory of Biology and Molecular Typing in Microbiology, University of Abomey-Calavi, Cotonou, Benin
6Department of Histology, Embryology, Cytogenetics and Human Reproduction (LHECHR), UFR Medical Sciences, University of Alassane Ouattara, Bouaké, Cote D'Ivoire
Received: 24-Mar-2023, Manuscript No. JBP-23-20278; Editor assigned: 28-Mar-2023, Pre QC No. JBP-23-20278 (PQ); Reviewed: 11-Apr-2023, QC No. JBP-23-20278; Revised: 18-Apr-2023, Manuscript No. JBP-23-20278 (R); Published: 25-Apr-2023, DOI: 10.35248/2155-9597.23.14.460
Abstract
The pathogenicity of P. aeruginosa, which is associated to acute and chronic infections, is related to its ability to form biofilms, and produce spoilage determinants such as pyoverdin, proteases, lipases, and toxins. The purpose of this research was to determine the involvement of pyoverdin, proteolytic and lipolytic enzymes in biofilm formation. A total of 33 strains of P. aeruginosa of animal (11), environmental (11) and clinical (11) origin were identified by PCR followed by sequencing. The microplate method was used to quantify biofilm formation. The pyoverdin on medium was determined by spectrofluorometry. The production of protease and lipase was sought respectively on agar with milk and with Rhodamine B on solid Luria-Bertani medium. In decreasing order of importance, the median value of biofilm formed was 1.5 (environmental strains); 1.3 (clinical strains) and 1.2 (animal strains). Biofilm categories were categorized into strong (37% to 48%), moderate (28% to 45%), and weak (6% to 14%) producers. The average pyoverdin production was 1304.4 nm, 1325.1 nm and 999.1 nm respectively in animal strains, environmental clinics. The production of protease ranged from 85.0% to 100% while that of lipase ranged from 70.0% to 90.6%. Control of certain virulence factors may reduce the pathogenicity of P. aeruginosa.
Keywords
P. aeruginosa; Biofilm; Pyocyanin; Protease; Lipase
Introduction
Pseudomnas aeruginosa is an opportunistic pathogenic bacterium responsible for acute and chronic infections [1]. Its activity essentially allows the passage from the commensal state to the pathogenic state.
Among the compounds involved in virulence in P. aeruginosa, proteases and lipases play an important role in acute infections [2], Indeed, in P. aeruginosa, the proteolytic and lipolytic enzymes produced by the quorum-sensing represent important virulence factors that have the capacity to alter host defenses [3]. Pseudomnas aeruginosa produces a multitude of extracellular proteases essential for the invasion of acute infections including LasA and LasB elastases [4]. LasA and LasB elastases are secreted under the regulation of Quorum Sensing (QS) systems and degrade host elastin [4]. LasB elastase, also called “elastase” or “pseudolysin”, is the most abundant protease and is the main factor of extracellular virulence [5].
In addition to proteases, lipases secreted by P. aeruginosa cleave lipids, producing free fatty acids and glycerol as end products [6]. LipA is the main lipase produced by P. aeruginosa and requires the lif chaperone for its activation [7]. These enzymes can be expressed under varying environmental conditions and influence other virulence factors [8,9].
Besides their involvement in the pathogenicity of P. aeruginosa; lipases and proteases are the most sought-after thermophilic enzymes in various industrial sectors, in particular because of their greater resistance to thermal and chemical denaturation [10]. Indeed, their multiple properties make them ideal candidates in biotechnology and generally in the Agro-food industries and in Organic synthesis [10,11]. These enzymes secreted by certain microorganisms and in particular P. aeruginosa produce compounds which affect the organoleptic quality and the market ability of certain local food products (bad taste, texture, or functional properties) [10,12].
P. aeruginosa also secretes two main siderophores, pyoverdin and pyochelin, which participate in bacterial virulence and biofilm development. These siderophores are small molecules that chelate iron from the environment for use in the bacteria's cellular metabolism. Since iron is an important element for the growth of P. aeruginosa, pyoverdin is therefore essential for virulence and bacterial growth under conditions of iron restriction. Pyoverdin regulates its own secretion as well as that of other virulence factors such as exotoxin A, a translation inhibitor, and the protease PrpL [13,14]. Pyoverdin also disrupts the host's iron and mitochondria homeostasis, even in the absence of the pathogen [13,15].
All these alteration determinants facilitate the establishment of the biofilm via quorum sensing, which is an interbacterial communication system. This is because biofilm formation is a cycle in which organized communities of bacteria are enclosed in a matrix of Extracellular Polymeric Substances (EPS) that hold microbial cells together at a surface [16,17]. Currently, around 80% of all microbial infections are associated with biofilms, of which 60%-70% are nosocomial infections caused by biofilms on contaminated medical material [18]. Various studies indicate that biofilm lifestyle, structure and composition lead to increased resistance to antimicrobial agents [19].
This is because biofilm bacteria resist the host's immune response and may be 10 to 1000 times more resistant to antimicrobial agents than planktonic bacterial cells [20]. The control of certain alteration determinants involved in the formation of biofilms therefore turns out to be a major public health issue. The objective of this work is to show the involvement of pyoverdin, proteolytic and lipolytic enzymes in the formation of the biofilm.
Materials and Methods
Isolation and identification of P. aeruginosa
Thirty-three (33) isolates of P. aeruginosa were obtained from animal products (smoked fish, fresh fish and beef) the environment (stalls, cutting instruments) and clinical samples (smoked fish, fresh fish and beef). Strains of P. aeruginosa were isolated and identified with the basis of standard microbiological techniques. Oxidase positive strains producing both types of pigment (pyocyanin and pyoverdin) on King A and King B or only pyocyanin on King A, and able to grow at 43°C were retained as P. aeruginosaafter molecular confirmation.
The oxidase positive strains, not producing pyocyanin and which cannot grow at 43°C, were considered as Pseudomonas sp and excluded from the retained strains. The molecular identification and sequencing of P. aeruginosa strains were carried out using the 16S gene as described in our recent work [21].
Biofilm formation by the microplate method
Biofilm formation in P. aeruginosa was quantified in 96-well polystyrene microplates according to the method described by Sabaeifard et al., with some modifications (Figure 1) [22]. Different suspensions with a final volume of 1.2 mL consisting of LB medium diluted 1:10 and Casamino acid with a final concentration of 0.5% are made in different Eppendorf tubes for each strain of P. aeruginosa.
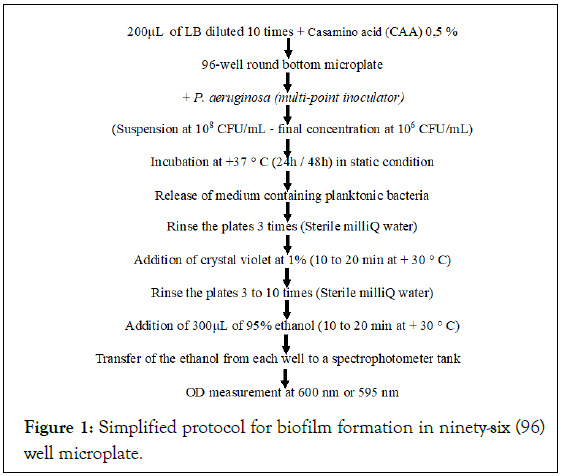
Figure 1: Simplified protocol for biofilm formation in ninety-six (96) well microplate.
Overnight cultures were adjusted to 0.5 McFarland (108 CFU/ mL). Each strain was tested in 5 replicates after inoculation of a culture standardized in Tryptic Soy Broth (TSB) supplemented with 0.2% glucose. Negative control wells contained Trypticase Soy Broth (TSB) not inoculated with glucose. Samples (200 μL) were distributed into wells, and the microplates were covered and incubated aerobically without shaking at 37°C for 48 hours.
After 48 hours, the bacterial suspension was aspirated and each well was washed three times with Phosphate Buffered Saline (PBS) (Sigma). The plates were dried at room temperature, stained with 200 μL of 1% (v/v) crystal violet solution for 20 min to 30 min (Figure 1). The excess crystal violet is removed by 3 to 10 successive manual washings of the plates with milliQ water and dried at room temperature.
The biofilm was fixed with 300 μL of ethanol (95%) for 15 min and was then removed. The dye bound to the adherent cells was resolubilized with 160 μL of 33% (v/v) glacial acetic acid (Sigma) per well. The concentration or Absorbance of the Dye (AD) is measured with a spectrophotometer at a wavelength of 595 nm with the CytationTM 5 cell imaging multimode reader (BioTek Instruments, Innovation, CA), linked to the software of Data Gen 5 analysis v. 2.04TM (BioTek Instruments, Innovation, California).
Finally, the strains were grouped into: OD595<0.1, Non-Producing (NP); OD595=0.1-1.0, Weak Producers (WP); OD595=1.1-3.0, Moderate Producers (MP); and OD595>3.0, Strong Producers (SP).
Pyoverdin production and quantification
The production of the fluorescent pigment (pyoverdin) was investigated by cultivating the strains of P. aeruginosa on King B (KB) medium as described by Nagata et al. (2013). After incubation at 37 °C for 24 hours, the production of fluorescent pigment was revealed with the naked eye or under a UV lamp at wavelengths of 254 nm and 365 nm (UVP, inc. Chromato-Vue UV Viewing System). Pyoverdin was quantified by dispensing different volumes of 200 μL of Trypticase Soy Broth (TSB) into the wells of 96-well black plates. Black plates were used to avoid cross-fluorescence from adjacent wells. Three replicates were performed for each strain. These different volumes (200 μL) were inoculated from culture stocks kept at -80°C in TSB+glycerol (15%) and incubated at 37°C with shaking.
The different values of pyoverdine were determined by spectrofluorometry (Biotek, Cytation imaging reader, Canada) at a wavelength of 600 nm with an excitation of 398 and an emission of 455.
Detection of proteolytic activity: Detection of caseinase
The hydrolysis of casein was demonstrated on an agar medium containing 1% milk (10 g/L), 2 g/L of yeast extract, 1.5% Agar as described by Andreani et al., [10]. After drying, the agars were inoculated with 5 μL of 24 hours young culture of TSB broth. After 24 hours of incubation at 37°C, the presence of the protease is detected by a clear halo around the colony indicating the hydrolysis of the casein. In contrast, a negative result shows no zone of hydrolysis around the culture.
Detection of lipolitic activity with Rhodamine B
The production of lipase was sought on solid Luria-Bertani (LB) medium as described by Patcha and Wiyada [23]. After autoclaving the LB medium (20 minutes, 121°C, 15 psi), a volume of 1% (v/v) olive oil (Selection Merite, Extra Virgin, 100% pure, bottled in Italy, imported for BRISKA, Montreal, QC) and 0.002% (2 mg/L) of Rhodamine B (Sigma) were added to the medium slightly cooled (~ 60°C). The whole was homogenized by a mixer (blender) and then 250 mL of this mixture was poured into plates under a biological hood (ESM). After drying, the agars were inoculated with 5 μL of 24 hours young culture of TSB broth and incubated at 37°C for 24 hours. Positive reactions result in the appearance of clear halo around the colony observed with the naked eye or under a UV lamp at wavelengths of 254 nm and 365 nm (UVP, Inc. Chromato- Vue UV Viewing System).
Statistical analysis
Data were entered with SPSS Statistics 20.0 data processing software (IBM Corporation, SPSS Inc, Chicago, USA) and transferred to Excel. Data were entered with Data Gen 5 v. 2.04™. The data were analyzed by t-test at a statistical level of α<0.05 (Excel, MS office).
Results
Biofilm formed by P. aeruginosa and categories of producers
The study demonstrated the ability of P. aeruginosa strains of animal, environmental and clinical origin to form biofilms within 48 hours. The median biofilm formation formed in 48 hours varies from 1.2 to 1.5 (Figure 2). Some strains were non-producing (Figure 3) and others producing biofilm, classified as strong (37% to 48%), moderate (28% to 45%) and weak (6% to 14%) producers (Figure 4).
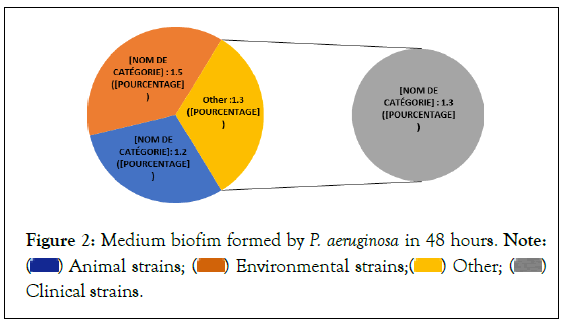
Figure 2: Medium biofim formed by P. aeruginosa in 48 hours. Note:  Clinical strains.
Clinical strains.
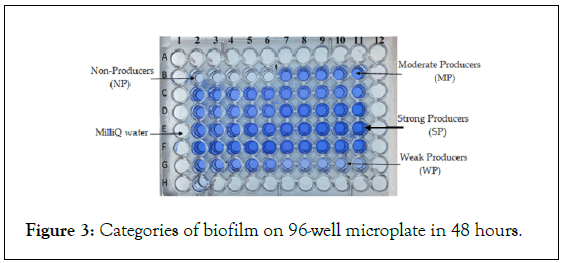
Figure 3: Categories of biofilm on 96-well microplate in 48 hours.
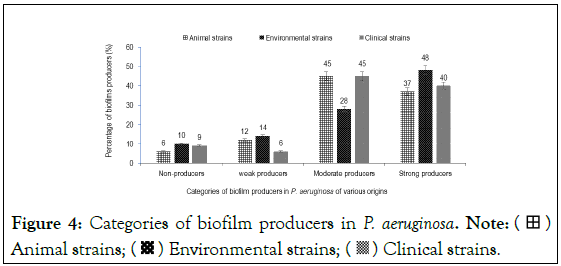
Figure 4: Categories of biofilm producers in P. aeruginosa. Note:  Animal strains;
Animal strains;  Environmental strains;
Environmental strains;  Clinical strains.
Clinical strains.
Production of pyoverdin in Pseudomonas aeruginosa
The results indicated an average pyoverdin production of 1304.4 and 1325.1 nm respectively in animal and clinical strains after growth of 24 hours (Figure 5). This average production of pyoverdin in environmental strains (999.1 nm) is lower than that produced by the reference strain P. aeruginosa PA 14 (1250 nm) (Figure 5). The minimum and maximum pyoverdin production was 20.9 (animal strains) and 2988.5 (clinical strains), respectively (Table 1). The Figure 6 shows the fluorescence and production of specific pigments in P. aeruginosa strains.
| Pyoverdin Excitation:398; Emission:455; DO:600 nm | Minimum pyoverdin | Medium pyoverdin | Maximum pyoverdin |
|---|---|---|---|
| Animal strains | 20.9 | 1304.4 | 8991.1 |
| Environmental strains | 407.9 | 999.1 | 2050 |
| Clinical strains | 457.1 | 1325.1 | 2988.5 |
Table 1: Average pyoverdin production in P. aeruginosa Culture for 24 hours at 37°C in LB medium contained in black 96-well round-bottom microplates under shaking conditions.
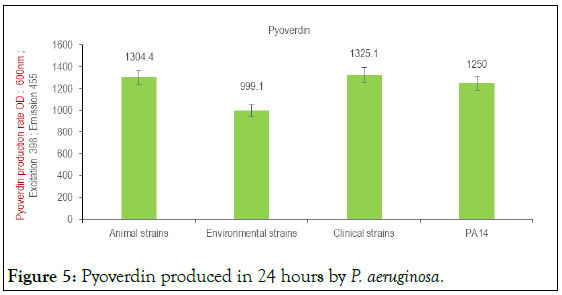
Figure 5: Pyoverdin produced in 24 hours by P. aeruginosa.
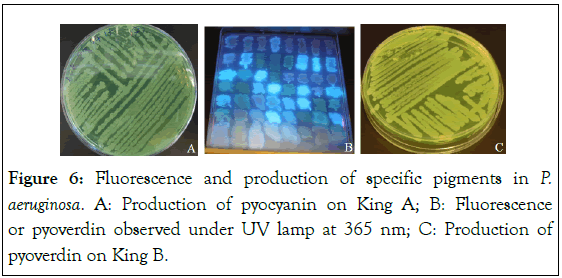
Figure 6: Fluorescence and production of specific pigments in P. aeruginosa. A: Production of pyocyanin on King A; B: Fluorescence or pyoverdin observed under UV lamp at 365 nm; C: Production of pyoverdin on King B.
Protease and lipase produced in P. aeruginosa
The production of protease and lipase is respectively higher in strains of P. aeruginosa of animal origin (97.5%;90.6%) and human (100%;88.8%) than in environmental strains (85.0%;70.0%) (Figure 7). The Figure 8 shows the production of protease and lipase in P. aeruginosa strains.
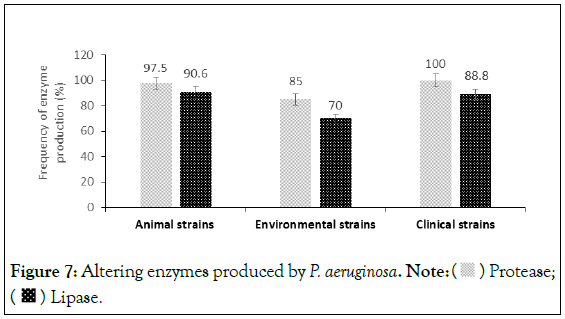
Figure 7: Altering enzymes produced by P. aeruginosa. Note:  Protease;
Protease;  Lipase.
Lipase.
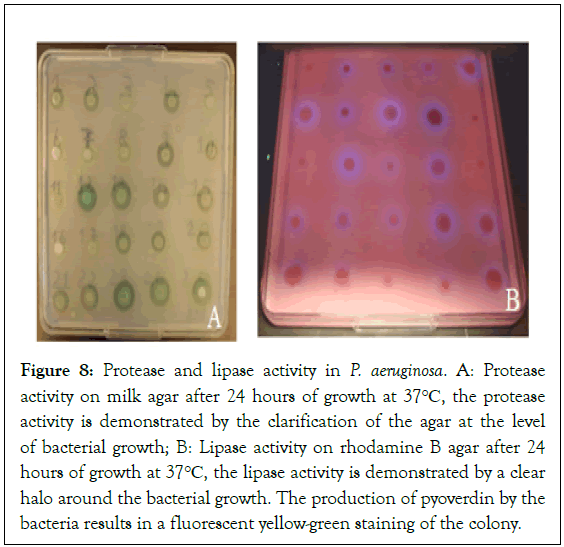
Figure 8: Protease and lipase activity in P. aeruginosa. A: Protease activity on milk agar after 24 hours of growth at 37°C, the protease activity is demonstrated by the clarification of the agar at the level of bacterial growth; B: Lipase activity on rhodamine B agar after 24 hours of growth at 37°C, the lipase activity is demonstrated by a clear halo around the bacterial growth. The production of pyoverdin by the bacteria results in a fluorescent yellow-green staining of the colony.
Discussion
In P. aeruginosa, certain extracellular virulence factors such as proteolytic, lipolytic enzymes, siderophores (pyoverdin and pyocyanin) and rhamnolipids are involved in the alteration and formation of the biofilm.
Biofilm formation remains a major public health problem [18]. Indeed, one of the peculiarities of P. aeruginosa forming biofilms is the loss of sensitivity to antimicrobials [24]. This study therefore demonstrated the ability of P. aeruginosa strains of animal, environmental and clinical origin to form biofilms within 48 hours.
The average percentages of biofilm formed in 48 hours shows that most strains were biofilm producers, classified as strong, moderate, and weak producers [25]. This biofilm formation could be promoted by cell motility, especially when it is mediated by flagella. Under certain environmental conditions, flagella are required for biofilm formation in P. aeruginosa.
In addition, it is different categories of biofilm formation could be due to bacterial surface appendages which can be weather dependent. In addition, the average number of adhered cells is higher in environmental strains (1.5) than in clinical (1.3) and animal (1.2) strains. These results show that strains of environmental origin may be more resistant to antimicrobial treatments than animal and clinical strains. Several studies have shown that the composition and availability of nutrients in the environment can promote the formation of biofilms [24-26].
Indeed, during their growth, bacteria produce homoserine lactones which are involved in the communication system called quorum sensing [27]. The accumulation of these molecules in the environment promotes the formation of biofilm.
The results of this study also indicated an almost identical mean pyoverdin production in animal and human strains of P. aeruginosa after 24 hours growth. This pyoverdin production is lower than the average 24-hour pyoverdin production in P. aeruginosa of environmental origin. The production of pyoverdin by strains of P. aeruginosa could contribute to the spoilage of food products and mainly animal products [28].
The production of pyoverdin observed in human strains could promote the mechanisms involved in the pathogenicity of P. aeruginosa by the phenomenon of Quorum sensing [29]. Indeed, Quorum-Sensing (QS) is a system of inter-bacteria communication and regulation of the transcription of virulence genes in P. aeruginosa.
In addition to the production of pyoverdin, P. aeruginosa produces lipases and proteases which are the most sought-after thermophilic enzymes in various industrial sectors, in particular because of their greater resistance to thermal and chemical denaturation [10,12].
Indeed, their multiple properties make them ideal candidates in biotechnology and generally for the agro-food industries and organic synthesis [10,11]. However, these enzymes secreted by certain microorganisms produce compounds which affect the organoleptic qualities and the marketability of certain local food products (bad taste, texture or functional properties) [10,12].
The results of this study indicate that proteases and lipases produced by bacteria, in particular Pseudomonas, could intervene respectively in the formation of volatile amines, ammonia and free fatty acids [10,23]. Indeed, on the one hand, these acids are the source of unpleasant odors and flavors and of the toxicity of the food [12,23].
In addition, they modify the technological and taste properties of foods, with the appearance of rancid taste [30]. Also, these acids promote the phenomenon of oxidation of unsaturated fatty acids to methyl ketones [11]. These results are consistent with those of several authors who have shown that Pseudomonas plays an important role in the spoilage of animal products such as dairy products [10,30]. Indeed, they pointed out that bacteria of the genus Pseudomonas produce thermo-tolerant lipolytic and proteolytic enzymes that reduce both the quality and shelf life of food.
In addition, the proteases and lipases produced by Pseudomonas aeruginosa promote the invasion and spread of the bacteria by causing the destruction of intercellular bonds by hydrolysis of proteins and lipases of the extracellular matrix [10,12,31]. The results showed that the enzymes produced by the bacteria come from a variety of sources.
Thus, the production of protease and lipase is higher in strains of P. aeruginosa of animal and human origin than in environmental strains. This diversity in the production of these enzymes could be explained by their presence in the form of extracellular proteins, both in invertebrates and in vertebrates but also in many microorganisms mainly [10,11].
Conclusion
The study revealed the ability of P. aeruginosa strains to form biofilms. It has also shown that proteolytic and lipolytic enzymes as well as pyoverdin are involved in the pathogenicity, in the alteration potential and in the virulence of P. aeruginosa. Procedures and techniques for the reliable and adequate preservation of food and mainly animal products should be applied in the processing chain.
Funding
This research did not receive any specific grant.
Declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Acknowledgments
The authors thank the INRS–Institut Armand Frappier of Canada, the Institut Pasteur of Paris, the Institut Pasteur of Côte d’Ivoire, and the Université Félix Houphouët Boigny for their excellent technical assistance.
References
- Rasamiravaka T, Labtani Q, Duez P, El J.M. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. BioMed Res Int. 2015:1-17.
[Crossref] [Google Scholar] [PubMed]
- Sultan M, Arya R, Kim KK. Roles of two-component systems in Pseudomonas aeruginosa virulence. Int J Mol Sci. 2021:1-22.
[Crossref] [Google Scholar] [PubMed]
- Rajkowska K, Otlewska A, Guiamet PS, Wrzosek H, Machnowski W. Pre-Columbian archeological textiles : A source of Pseudomonas aeruginosa with virulence attributes. Applied Sci. 2020;10:10-11.
- Ruffin M, Bilodeau C, Maille E, LaFayette SL, McKay GA, Thu Ngan Trinh N, et al. Quorum-sensing inhibition abrogates the deleterious impact of Pseudomonas aeruginosa on airway epithelial repair. FASEB J. 2016;30:3011-3025.
[Crossref] [Google Scholar] [PubMed]
- Wolf P, Elsasser-Beile U. Pseudomonas exotoxin A: From virulence factor to anti-cancer agent. Int J Med Microbiol..2009;299:161-176.
[Crossref] [Google Scholar] [PubMed]
- Bleves S, Viarre V, Salacha R, Michel GP, Filloux A, Voulhoux, R. Protein secretion systems in Pseudomonas aeruginosa: A wealth of pathogenic weapons. Int J Med Microbiol. 2010;300:534-543.
[Crossref] [Google Scholar] [PubMed]
- Verma N, Dollinger P, Kovacic F, Jaeger KE, Gohlke H. The membrane-integrated steric chaperone Lif facilitates active site opening of Pseudomonas aeruginosa Lipase A. J Comput Chem. 2020;41:500-512.
[Crossref] [Google Scholar] [PubMed]
- Bofill C, Prim N, Mormeneo M, Manresa A, Pastor FI, Diaz, P. Differential behaviour of Pseudomonas sp. 42A2 LipC, a lipase showing greater versatility than its counterpart LipA. Biochimie. 2010;92:307-316.
[Crossref] [Google Scholar] [PubMed]
- Rosenau F, Isenhardt S, Gdynia A, Tielker D, Schmidt E, Tielen P, et al. Lipase LipC affects motility, biofilm formation and rhamnolipid production in Pseudomonas aeruginosa. FEMS Microbiol Lett. 2010;309:25-34.
[Crossref] [Google Scholar] [PubMed]
- Andreani NA, Carraro L, Fasolato L, Balzan S, Lucchini R, Novelli E, et al. Characterisation of the thermostable protease AprX in strains of Pseudomonas fluorescens and impact on the shelf-life of dairy products: preliminary results. Ital J Food Saf.2016;5:239-244.
[Crossref] [Google Scholar] [PubMed]
- Kessler E, Safrin M. Elastinolytic and proteolytic enzymes. Methods Mol Biol. 2014:1149:135-169.
[Crossref] [Google Scholar] [PubMed]
- Dufour D, Nicodème M, Perrin C, Driou A, Brusseaux E, Humbert G, et al. Molecular typing of industrial strains of Pseudomonas spp. isolated from milk and genetical and biochemical characterization of an extracellular protease produced by one of them. Int J Food Microbiol. 2008;125:188-196.
[Crossref] [Google Scholar] [PubMed]
- Kang D, Kirienko DR, Webster P, Fisher AL, Kirienko NV. Pyoverdine, a siderophore from Pseudomonas aeruginosa, translocates into C. elegans, removes iron, and activates a distinct host response. Virulence. 2018;9:804-817.
[Crossref] [Google Scholar] [PubMed]
- Yang L, Nilsson M, Gjermansen M, Givskov M, Tolker Nielsen T. Pyoverdine and PQS mediated subpopulation interactions involved in Pseudomonas aeruginosa biofilm formation. Mol Microbiol,2009;74:1380-1392.
[Crossref] [Google Scholar] [PubMed]
- Kang D, Kirienko NV. An in vitro cell culture model for pyoverdine-mediated virulence. Pathogens. 2020;10:9.
[Crossref] [Google Scholar] [PubMed]
- Crespo A, Blanco Cabra N, Torrents E. Aerobic Vitamin B12 biosynthesis is essential for Pseudomonasaeruginosa Class II ribonucleotide reductase activity during planktonic and biofilm growth. Front Microbiol. 2018;9:1-10.
[Crossref] [Google Scholar] [PubMed]
- Abdullah AA, Algburi A, Huang Q, Ahmad T, Zhong H, Javed HU, et al. Anti-biofilm potential of elletaria cardamomum essential oil against Escherichia coli O157:H7 and Salmonella typhimurium JSG 1748. Front Microbiol. 2021;12:1-10.
[Crossref] [Google Scholar] [PubMed]
- Marques VF, Motta CCD, Soares BS, Melo DA, Souza MMS. Biofilm production and beta-lactamic resistance in Brazilian Staphylococcus aureus isolates from bovine mastitis. Braz J Microbiol. 2017;48:118-124.
[Crossref] [Google Scholar] [PubMed]
- Simöes M, Simöes LC, Vieira MJ. A review of current and emergent biofilm control strategies. Food Sci Technol. 2010;43:573-583.
- Ceri H, Olson ME, Turner RJ. Needed, new paradigms in antibiotic development. Expert Opin Pharmacother. 2010;11:1233-1237.
[Crossref] [Google Scholar] [PubMed]
- Benie CKD, Attien YP, Tra Bi YC, Atobla K, Dadie A. Biofilm formation and inhibitory effect of essential oils in multidrug-resistant Pseudomonas aeruginosa. J Bacteriol Mycol: Open access. 2021;8:1192.
- Sabaeifard P, Abdi-Ali A, Soudi MR, Dinarvand R. Optimization of tetrazolium salt assay for Pseudomonas aeruginosa biofilm using microtiter plate method. J Microbiol Methods. 2014;105:134-140.
[Crossref] [Google Scholar] [PubMed]
- Patcha B, Wiyada M. Lipase-producing bacterium and its enzyme characterization. J Life Sci Technol. 2013;1:196-200.
- Srey S, Jahid IK, Ha SD. Biofilm formation in food industries: A food safety concern.Food Cont. 2013;31:572-585.
- Rossi C, Chaves López C, Serio A, Goffredo E, Goga BTC, Paparella A. Influence of incubation conditions on biofilm formation by Pseudomonas fluorescens isolated from dairy products and dairy manufacturing plants. Ital J Food Saf. 2016;5:154-157.
[Crossref] [Google Scholar] [PubMed]
- Ansari F, Ahmad I. Biofilm development, plant growth promoting traits and rhizosphere colonization by Pseudomonas entomophila FAP1: A promising PGPR. Adv Microbiol. 2018;8:235-251.
- Garcia Contreras R, Maeda T, Wood TK. Resistance to quorum-quenching compounds. Appl Environ Microbiol. 2013;79:6840-6846.
[Crossref] [Google Scholar] [PubMed]
- Al Kilabi AAK, Al Turaihi TS, Al Mohammed HS. Molecular detection of Quorum sensing genes in Pseudomonas aeruginosa isolated from CSOM patients and their relationship to biofilm ability. Eurasian J Biosci. 2020;14:4929-4934.
- Nagata T, Oobo T, Aozasa O. Efficacy of a bacterial siderophore, pyoverdine, to supply iron to Solanum lycopersicum plants. J Biosci Bioeng. 2013;115:686-690.
[Crossref ] [Google Scholar] [PubMed]
- Maisuria VB, Santos YL, Tufenkji N, Déziel E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci Rep. 2016;6:30169.
[Crossref] [Google Scholar] [PubMed]
- Periasamy A, Myoung Ju N, Da Hye K, Jun Seok S, Byung Ki H, Kyeong H. Screening and optimization of extracellular lipases by Acinetobacter species isolated from oil-contaminated soil in South Korea. African J Biotechnol. 2011;10:4147-4156.
Citation: Attien YP, Benié CKD, Sina HO, Tuo WTA, Zebre A, Kouassi CK, et al. (2023) Involvement of Pyoverdin, Proteolytic and Lipolytic Enzymes in Pseudomonas aeruginosa Biofilm Formation. J Bacteriol Parasitol. 23:460.
Copyright: © 2023 Attien YP, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

