Indexed In
- Open J Gate
- JournalTOCs
- The Global Impact Factor (GIF)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 9, Issue 5
Investigating the Impact of Existing Pharmacovigilance Systems in Africa and How They Influence Adverse Drug Reaction Reporting by Nurses, Doctors and Pharmacists Working in Federal Government Hospitals - A Case Study of Nigeria
Larry L Mweetwa1*, Bwalya A. Witika2, Kabo O. Tshiamo3, Paul Chukwuemeka Adiukwu4, Thatoyaone J Kenaope5, Emmanuel Tope Oluwabusola6 and Pedzisai A. Makoni7*2ApotheCom A MEDiSTRAVA Company (Medical Division of Huntsworth), London, United Kingdom
3Nathan and Family Pharmacy, Gaborone, Botswana, South Africa
4School of Pharmacy, University of Botswana, Gaborone, Botswana, South Africa
5Department of Pharmacy, Boitekanelo College, Gaborone, Botswana, South Africa
6Marine Biodiscovery Centre, University of Aberdeen, United Kingdom
7Division of Pharmacology, Faculty of Pharmacy, Rhodes University, Makhanda, South Africa
Received: 08-Apr-2021 Published: 29-Apr-2021, DOI: 10.35248/2329-6887.21.9.314
Abstract
Background: The primary concern of Pharmacovigilance (PV) is to strengthen patient care and improve public safety in terms of effective use of medication. The aim of this research was to analyse the impact of the existing Pharmacovigilance systems in the African region and its significant impact on adverse drug reactions (ADRs) reported by the Doctors, Pharmacists, and Nurses working in Federal-State Hospitals in Nigeria.
Methods: The research design opted for this study is constructed on a questionnaire-based quantitative design that has helped in determining the correlation between Healthcare Professionals (HCP) training and awareness of ADR reporting incidences in different federal government hospital settings of Nigeria.
Results: The Pearson chi-square test of independence with df = 2 yields chi-square = 101.4 (n = 318, p < 0.05, phi = 0.565), affirming that there is a statistically significant relationship between the awareness of ADR-reporting system and professions.
Conclusion: The findings revealed that a majority of pharmacist and doctors possessed a good knowledge regarding PV, whereas the majority of nurses had poor knowledge about PV.
Keywords
Pharmacovigilance; Adverse Drug Reactions; Knowledge; Attitude; Practice; Nigeria; KAP.
Introduction
According to the definition provided by the World Health Organization (WHO), Pharmacovigilance (PV) is referred to as science and activities performed to identify, detect, evaluate, comprehend, and prevent the adverse reactions that resulted due to the drug-related problem [1,2]. The primary concern of the PV is strengthening patient care and improving public safety in terms of the efficacy of medications. The PV is also aimed at supporting public awareness regarding the programs of promoting knowledge regarding the risk-benefit assessment profiles of multiple drugs to mitigate the irrational utilisation of medication [3]. The Adverse Drug Reactions (ADR) have become a global concern and has been causing a significant economic burden on the healthcare systems, and the analysis of the potential reasons of ARD revealed that limited awareness regarding the magnitude, as well as consequences of the ADR-related inaccuracies, are some of the most efficient reasons of an increment in the spread of pandemic situations, specifically within Africa [4].
ADRs are one of the globally recognised healthcare issues across the globe and are required to be catered seriously at all healthcare levels. Due to increased rates of mortality during the pandemic situations in Africa, the region has been introduced to the newly developed medications, which are critically monitored for promoting safety as well as the efficacy of medications [5]. The scopes, as well as severity of the PV activities, as well as mismanagement of ADR symptoms, have been resulting in a significant increment in the rates of hospitalisation in African countries. The lack of ADR reporting reduces the prospects related to the identification of the efficient drug regimen, as well as alternative therapies [6].
Agu and Oparah [7], analysing with reference to the Nigerian healthcare sector, the lack of awareness and knowledge among the healthcare providers are one of the most significant concerns, which has been resulting in a limited reporting of ADR cases by Federal Government Hospitals of Nigeria. With reference to Nigeria, National Pharmacovigilance Centre, governed by the National Agency for Food and Drug Administration and Control (NAFDAC), regulates the PV system and ensures the right use and safety of medicinal products [8]. The NAFDAC units were created for monitoring PV activities within the countries [9]. The formation of regional centres was performed for decentralisation of the activities offered by the NPC with reference to the distribution, as well as the collection of ADR forms, as well as ICSRs from the healthcare institutions for promoting the preliminary examination, as well as reporting of the healthcare-related issues [10]. The regional centres also monitor the PV activities at the institutional level and instigate an ownership sense within the healthcare professionals for integrating the ADR reporting within their practice.
Under the NPC, ADR reporting forms have been developed along with the guidance documents for facilitating the healthcare professionals to report the ADR [8]. NPC has initiated training programs for healthcare professionals. With the increment in recognition of PVC, NPC has also been focusing on dealing with the dealers of patient medicines for improving medication-related research and development in Nigeria. The healthcare providers have been reported to play a significant role in assessing, detecting, reporting, as well as monitoring ADR-related reports [11]. In this regard, improvement in knowledge, attitudes, as well as perceptions of the HCPs is likely to promote PV efficiency. However, with reference to Nigerian healthcare, the lack of training of healthcare professionals, the unavailability of training opportunities for the healthcare professionals, the unavailability of the reporting tools and techniques, increased workload, as well as the incurred cost is some of the factors responsible for limited reporting of ADR within the hospital settings of Nigeria [12]. The aims of this research were the assessment of current levels of knowledge, as well as the growth of the PV among the healthcare professionals in Nigeria, and evaluate the challenges regarding the implementation of robust as well as efficient drug surveillance systems. This research also aims to determine the causes of under-reporting of the drug-related adverse reactions in Nigeria. This research also analysed the roles, practices, as well as responsibilities of the NAFDAC for improving the healthcare reforms in Nigeria. The PV analysis policy has offered significant insight into understanding the challenges between existing healthcare professionals, as well as NAFDAC, and the manner in which the dug safety, quality control, care reporting, as well as regulatory decisions can be used for addressing the issues in relation to the ADR management of the registered nurses within Nigeria.
Method
The study investigates the impact of pharmacovigilance systems and their consequent impact on ADR reporting by the healthcare professionals working at the federal government hospitals of Nigeria.
Study Design
The selected research topic is investigated using a questionnairebased quantitative design to determine the correlation between health care professionals (HCP) training and awareness of ADR reporting incidences in federal government hospitals of Nigeria.
Study Population
The targeted population for the study includes the five federal government hospitals of Nigeria. The name of the hospitals under observation is Maitama, Kubwa, Wuse, Gwarinpa and Nyanya. The population also includes the doctors, nurses, and pharmacists collectively known as the chief healthcare professionals.
Sample Size
The total number of participants in the research (N) was 384. Around 400 questionnaires were distributed to healthcare professionals in the above mentioned five federal government hospitals of Nigeria. A total of 103 questionnaires were distributed among the doctors, 128 to nurses and 87 questionnaires to the pharmacists. Out of the total 400, 318 responses were filled accurately, making the research study sample size.
N=Z2PQ/D2
N= Target Population
Z= Standard Normal Deviation from the Confidence Level 1.96
P= Proportion of the Targeted Population being Measured 0.5
Q= 1-P 0.5
D= Level of Statistical Significance Set 0.05
N= (1.96)2 (.50)(0.05)2 384
Data Collection and Tool
There are two broad ways of collecting data for research, namely primary and secondary data collection method. The primary data collection method focuses on gathering first-hand data with the help of surveys and interviews. In contrast, the secondary data collection method focuses on collecting data from previous studies. For the research in hand, primary sources of data collection were used. The data has been collected through nonexperimental research methods that include surveys to correlate various factors of the research topic. For the data collection, a survey questionnaire with close-ended questions was designed to study the impact of pharmacovigilance (PV) systems and reporting of adverse drug reactions (ADR) by the healthcare professionals in the five federal government hospitals of Nigeria. To comply with the data's confidentiality concerns, the data collected was stored on a cloud-based encrypted file. The cloud-based data was kept password protected, shared with the supervisor, the researcher, and the authorised personnel involved in conducting the research. No third party had any access to the data.
Data Analysis
For the analysis of collected data, descriptive statistics are used to explore the relationship between the dependent and independent variables by using the parametric and non-parametric tools. For the research under study, different descriptive statistics, including bar charts, histograms, line graphs, and dispersion ranges, are selected. For charts and diagrams, SPSS 25.0 and MS Excel were used to determine the impact of PV activities on adverse drug reaction reporting by HCP in Kubwa, Gwarinpa, Wuse, Maitama and Nyanya setups in Nigeria.
Privacy and confidentiality are considered an essential pillar of ethical consideration for the study. The research's ethical consideration included approval from the University of Hertfordshire and the federal ethics committee of Nigeria. Consent forms were retrieved from the hospitals mentioned in the research. Furthermore, a clear understanding of the objectives and data collection process was ensured by the respondents. The outcomes and future prospects were discussed prior to the research, and the privacy, rights, and interests of participants were protected. The data was stored in a password protected cloud-based folder for ensuring privacy.
Results
Response Rate and Demographic Profile
The questionnaires were administered to HCPs, specifically doctors, nursing professionals, and pharmacists, present within the Federal Government hospitals of Nigeria. The hospitals working under the federal government included Gwarinpa, Wuse, Kubwa, Maitama, and Nyanya Hospitals. Initially, the total number of the HCPs working within the Federal Government Hospitals were recorded, and N= 295 (27%) doctors, N=107 (10%) pharmacists, as well as and N= 665 (62.3%) nurses working in FTC selected (Figure 1). The analysis of demographic information of the respondents revealed that out of N= 318 respondents, N=103 (32%) doctors, N=87 (27%) pharmacist, and N=128 (40%) nurses participated in the research. The information regarding the number and type of healthcare professionals working at the selected hospitals of Nigeria's considered regions (Figure 2).

Figure 1. HCPs demographics at hospitals selected in the study per FCT.
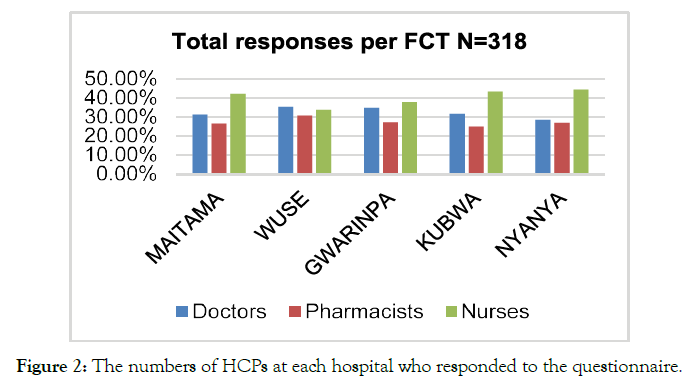
Figure 2. The numbers of HCPs at each hospital who responded to the questionnaire.
The analysis of the age of respondents revealed that a majority of the respondents belonged to an age group of 31-40 years; however, a considerable majority of nursing professionals, N=46 nurses, out of the total 35.9% were from the age group of 20-30 years (Figure 3). The analysis of the gender demographic information of the respondents mentioned that N= 171 were females, whereas N= 147 respondents were male. N= 47 were doctors, N= 95 were nurses, and N= 29 were pharmacists with reference to the female population. On the other hand, N= 58 were pharmacist, N= 56 were doctors, N=33 were nurses. In this regard, there was a considerably greater proportion of females among nurses than pharmacists and doctors (Figure 4).
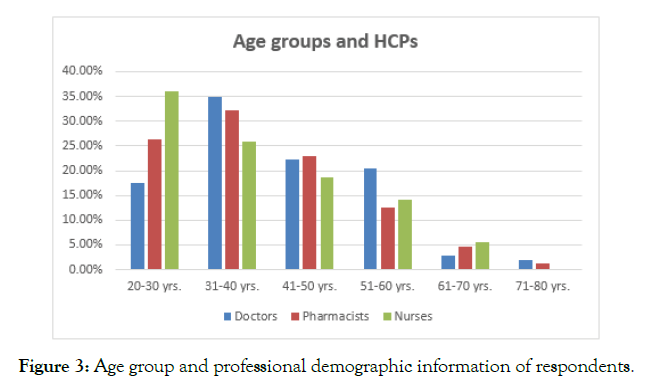
Figure 3. Age group and professional demographic information of respondents.
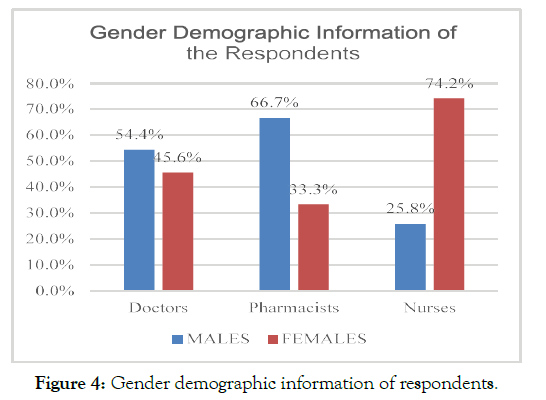
Figure 4. Gender demographic information of respondents.
Awareness of ADRs Reporting
The analysis of the opinions of respondents regarding the knowledge related to PV practices revealed that approximately 51.3% of the respondents mentioned that they report ADR cases to the ministry of health. The analysis of reporting preferences of the healthcare professionals revealed that 83.6% of the nurses and 54.4% of doctors reported that ADR reporting is required to be done to the ministry of health. On the contrary, 97.7% of the pharmacists emphasised that ADR reporting should be submitted to NAFDAC; rather than the ministry of health. Moreover, very few respondents, 3.12% of nurses, 8.7% doctors, and 2.2% pharmacists, reported that the ADR reports are required to be submitted to the Nigeria Food and Drug Control and Drug enforcement commission (Figure 5).
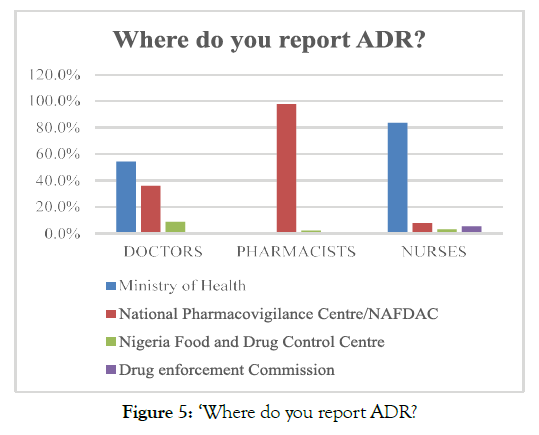
Figure 5. ‘Where do you report ADR?
The respondents were asked about the time duration in which it is essential to report the serious and unexpected ADRs to the concerned regulatory authorities of Nigeria. Less than half, i.e., N=123 (38.7%) of the respondents, mentioned that ADR reporting is required to do within the time duration of 15 days; however, N=114 (35.8%) of the respondents mentioned that ADR reporting must be carried out within the 7 days. Analysing an individual perspective of the staff, 59.4% of nurses believed that ADR is required to be reported within 7 days, whereas 35.9% mentioned that ADR should be reported within 10 days. On the contrary, 89.7% of the pharmacists mentioned that the ADR should be reported within 15 days. In this regard, the pharmacists were more aware of the duration in which ADR should be reported to the concerned authorities (Figure 6).
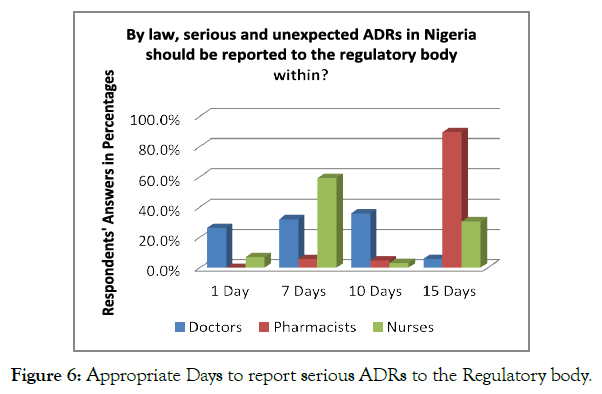
Figure 6. Appropriate Days to report serious ADRs to the Regulatory body.
In response to the questions regarding the ADR reporting can be considered as a professional obligation, 84.9% of the respondents considered the ADR reporting as their professional obligation. In contrast, only 3.8% of the considered healthcare professionals did not consider ADR as their professional obligation. Some of the nurses reported that ADR reporting is only the responsibility of pharmacists because they are custodians of medicines (Figure 7).
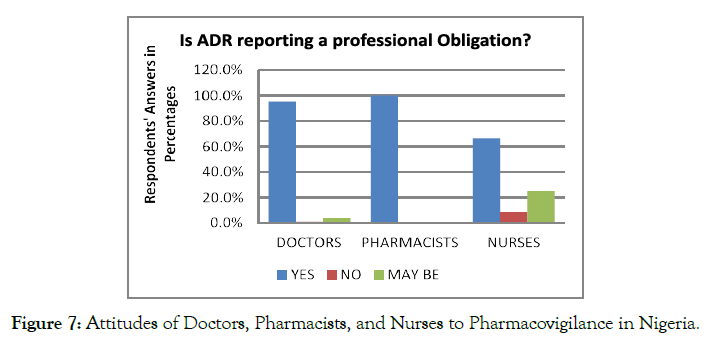
Figure 7. Attitudes of Doctors, Pharmacists, and Nurses to Pharmacovigilance in Nigeria.
When the respondents were asked regarding their awareness levels regarding the ADR systems, it was found that a considerable majority was not aware of ADR reporting systems. According to the statistical analysis, 98 nurses (76.6%) and 70 doctors (68%) denied having any information regarding ADR reporting systems of Nigeria, whereas 89.7% of the pharmacists were reported to possess efficient knowledge regarding the ADR reporting systems (Figure 8).

Figure 8. Awareness of ADR reporting system in Nigeria.
Association between Training in PV and Profession
The findings revealed that a majority of pharmacist and doctors possessed a good knowledge regarding PV, whereas the majority of nurses had poor knowledge about PV (Figure 9). The Pearson chisquare test of independence with df = 2 yields chi-square = 101.4 (n = 318, p < 0.05, phi = 0.565), affirming that there is a statistically significant relationship between the awareness of ADR-reporting system and professions (Table 1).
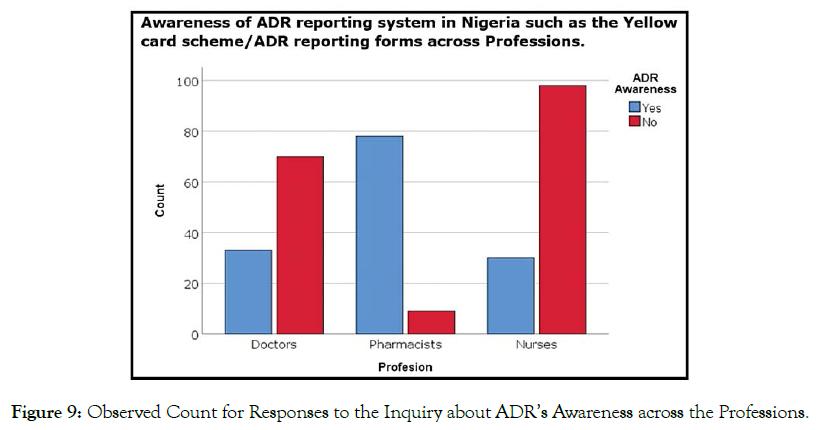
Figure 9. Observed Count for Responses to the Inquiry about ADR’s Awareness across the Professions.
| Profession ADR Reporting System awareness Crosstabulation | |||||
| ADR Awareness | |||||
| Yes | No | Total | |||
| Profession | Doctors | Count | 33 | 70 | 103 |
| Expected Count | 45.7 | 57.3 | 103.0 | ||
| Pharmacists | Count | 78 | 9 | 87 | |
| Expected Count | 38.6 | 48.4 | 87.0 | ||
| Nurses | Count | 30 | 98 | 128 | |
| Expected Count | 56.8 | 71.2 | 128.0 | ||
| Total | Count | 141 | 177 | 318 | |
| Expected Count | 141.0 | 177.0 | 318.0 | ||
Table 1: Observed and Expected Counts - Representing Chances of Relationship between Variables.
Analysis of ADR Reporting by Profession
In response to whether the participants report ADR to the concerned authorities, N=163 (51.3%) replied in affirmative, whereas N=155 (48.7%) mentioned that they do not report ADR events to the concerned authorities. With reference to the considered professions, N=86 (83.5%) of doctors and N=56 (64.4%) of pharmacists reported that they had reported adverse drug reactions during their professional practice (Figure 10).
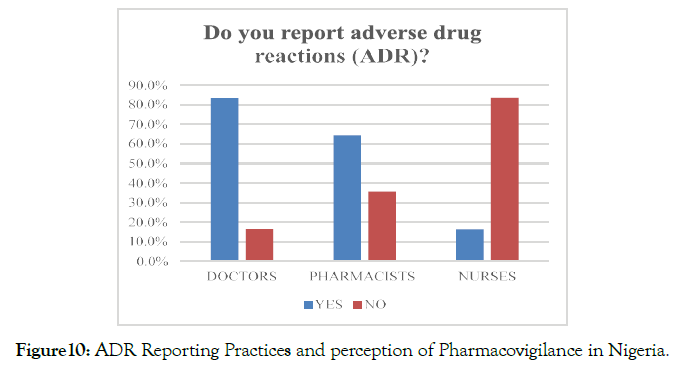
Figure 10. ADR Reporting Practices and perception of Pharmacovigilance in Nigeria.
A considerable number of the respondents, N=98 (95.1%) and N=124 (96.9%) reported that they had not reported any ADR cases, whereas N=87 (72.4%) of the pharmacists were found to report the ADR events to the concerned authorities (Figure 11).
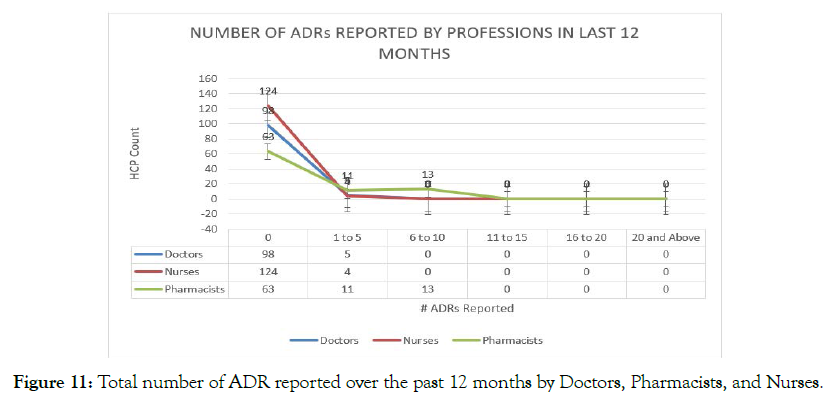
Figure 11. Total number of ADR reported over the past 12 months by Doctors, Pharmacists, and Nurses.
Factors Preventing HCPs from Reporting ADRs
There are several factors, which restricts the healthcare professionals from ADR reporting, such as awareness, the potential outcomes which might be achieved by ADR reporting, and lack of clarity regarding the ADR reporting with reference to their professional responsibilities. The analysis of factors restricting the healthcare professionals from ADR reporting revealed that 26.7% of the respondents were unsure about the mechanism to report the ADR cases. In addition, 18.2% of the healthcare professionals mentioned that n Nigeria, there is no enough information available for ADR reporting, which make it difficult for them to report the cases. Moreover, N= 24.5% of healthcare professionals reported that they do not have a noticeable benefit, feedback, or financial reward for ADR reporting, restricting them from ADR reporting. N= 35 (11%) of the respondents mentioned that reporting of ADR is neither their duty nor a legal requirement. However, 5.66% of the respondents mentioned that they were not sure what events should be reported and in which cases the medications cause ADRs. A small majority (2.51%) of the respondents also highlighted that ADR reporting was timeconsuming for them (Figure 12).
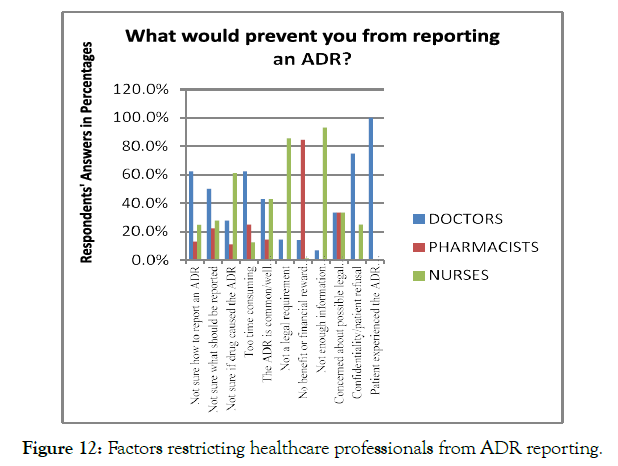
Figure 12. Factors restricting healthcare professionals from ADR reporting.
Other factors restricting the healthcare professionals of Nigeria from ADR reporting was PV training. Overall, 76.4% of the total respondents denied receiving the PV training, and only 23.5% of the respondents were trained for ADR reporting. Out of the healthcare professionals, the nurses were less trained regarding PVR than the doctors and pharmacists. Thus, the lack of proper education delivered to the Nigerian medical healthcare providers was one of the most significant reason, restricting them from ADR reporting (Figure 13).
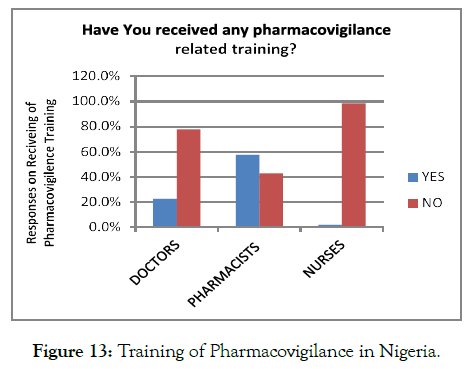
Figure 13. Training of Pharmacovigilance in Nigeria./p>
Another reason for not reporting ADR was that the healthcare professionals perceived that the reporting of ADR to the concerned authorities is the pharmacist's responsibility, which revealed that the healthcare professionals lacked appropriate education regarding the significance of ADR reporting. About 38.7% of the total healthcare professionals were reported to be extremely busy in their daily routine and do not have enough time to report ADRs, whereas 18.2% of the nurses proclaimed that during their professional practice, failure in determining the ADRs restricted them from reporting of ADR events (Figure 14).
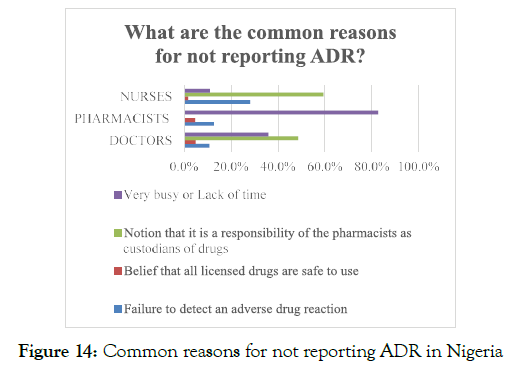
Figure 14. Common reasons for not reporting ADR in Nigeria
The respondents were asked what measures are required to be taken for making improvements in the ADR reporting systems in Nigeria, and it was found that more than half, 56.6% of the total respondents suggested that giving monetary benefit for reporting the ADR cases would encourage the healthcare professionals to report ADR to the concerned authorities. Moreover, 12.6% of the total respondents mentioned that improving the accessibility of the healthcare professionals towards the ADR reporting material, such as yellow card, might also improve the ADR reporting system in Nigeria (Figure 15). In addition, proper training and education in PV and the significance of PV reporting should also be provided for enhancing the ADR-related health services in Nigeria.
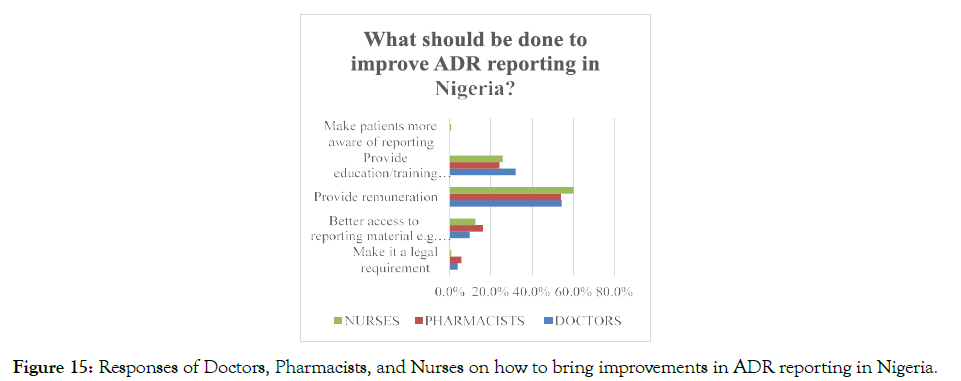
Figure 15. Responses of Doctors, Pharmacists, and Nurses on how to bring improvements in ADR reporting in Nigeria.
Discussion
Despite the concerns of the Nigerian healthcare authorities towards PV and ADR reporting, approximately 50% of the inpatients in Nigerian healthcare are at an increased risk of experiencing ADR [13]. The under-reporting of ADR has become an additional burden on the population of Nigeria, with reference to the scarcity in the preventive measures, contributing to the hazardous consequences. The ADR reporting requires voluntary submission of the patientspecific data in relation to the suspected ADR to the concerned drug regulated agency before administrating any other medicinal product. The growth of PV is significantly dependent on the frequency of ADR cases; however, it was noticed that considerably few healthcare professionals in Nigeria report the ADR incidences to the concerned authorities. Regarding the levels of awareness of the healthcare professionals towards PV and ADR reporting, it was found that a considerable majority was not sure regarding the concerned authorities for ADR reporting, and half of the respondents (mostly medical doctors) were found to report ADR cases to the ministry of health, whereas, pharmacists proclaimed that ADR should be reported to NAFDAC; rather than the ministry of health. A few respondents also indicated that ADR reports are required to be submitted to the Nigeria Food and Drug Control and Drug enforcement commission. The awareness levels of the Nigerian healthcare professionals regarding the ADR reporting was also analysed with reference to the time duration in which SDR should be reporting to the concerned authorities. Less than half of the respondents mentioned that ADR reporting is required to be done within the time duration of 15 days; however, 35.8% of the respondents mentioned that ADR reporting must be carried out within 7 days. In addition, some of the healthcare professionals proclaimed that ADR reporting should be done within 10 days of the event. However, the analysis of the knowledge level of the healthcare professionals revealed that the pharmacists possessed more knowledge and awareness regarding ADR reporting. The analysis of the responses provided by the respondents revealed that it is a common perception among the healthcare professionals that ADR reporting is only the responsibility of pharmacists because they are custodians of medicines. The association between the training and awareness related to PV and ADR reporting revealed that pharmacists were more trained in PV than medical doctors and nursing professionals. The outcomes of this research are in accordance with the research performed by Ezuko [14], who mentioned that the healthcare professionals are required to trained and knowledgeable regarding the ADR reporting systems for improving the detection, as well as the frequency of reporting. However, the healthcare professionals who have been trained in PV reporting are more likely to report these events to the concerned authorities than others. Ezuko [14] also mentioned that due to a lack of awareness, healthcare professionals consider it unnecessary to report ADR. It was found that ARD reporting could be improved in Nigeria after the placement and enforcement of the PV systems; however, it is essential for easing the process for the staff and encouraging them to report ADR.
There are several factors, which restricts the healthcare professionals from ADR reporting, such as awareness, the potential outcomes which might be achieved by ADR reporting, and lack of clarity regarding the ADR reporting with reference to their professional responsibilities. The statistical analysis revealed that 26.7% of the respondents were not sure about the mechanism to report the ADR cases, whereas 18.2% of the healthcare professionals mentioned that in Nigeria, there is no enough information available for ADR reporting, making it difficult to report the cases. Moreover, N= 24.5% of healthcare professionals reported that they do not have a noticeable benefit, feedback, or financial reward for ADR reporting, restricting them from ADR reporting. Other factors restricting the healthcare professionals of Nigeria from ADR reporting was PV training. Overall, 76.4% of the total respondents denied receiving the PV training, and only 23.5% of the respondents were trained for ADR reporting. About 38.7% of the total healthcare professionals were reported to be extremely busy in their daily routine and do not have enough time to report ADRs, whereas 18.2% of the nurses proclaimed that during their professional practice, failure in determining the ADRs restricted them from reporting of ADR events. Thus, the lack of proper education delivered to the Nigerian medical healthcare providers was one of the most significant reasons restricting ADR reporting.
The limitations associated with the research study were time and geographical constraints that limited the survey to five hospital setups in Nigeria. The total number of Pharmacists in each facility was limited compared to the other healthcare professionals. In contrast, the number of doctors was comparatively more than the other two professions, despite the fact that the facilities were mapped adequately. Thus, the research outcomes might be biased due to the inclusion of an unequal number of healthcare professionals from different disciplines.
Conclusion
The study aimed to investigate the impact of pharmacovigilance systems in Africa and how they influence the adverse drug reaction reporting by nurses, pharmacists, and doctors working in the federal government hospitals of Nigeria. To analyse the impact of PV systems, the following objectives were formulated. To analyse the knowledge of healthcare professionals related to pharmacovigilance activities, study the role and importance of pharmacovigilance activities in Nigeria, determine the relationship between PV training and frequency of ADR reporting, and assess the initiatives of NAFDAC in improving the awareness and knowledge. The purpose of conducting the research was to fill up the knowledge gap in the field of medical health services to facilitate the growth of national pharmacovigilance systems in Nigeria. The research will provide technical support to Nigeria's government to take steps essential for improving the country's PV system.
Literature focused on several factors affecting the growth of pharmacovigilance systems in Nigeria. These include a lack of awareness regarding the importance of reporting ADR. The lack of awareness serves as the major contributor to poor pharmacovigilance performance in the country as put forth by the world health organization’s monitoring facility in Uppsala. However, it was established that proper education regarding pharmacovigilance could result in better health care services in the country. Furthermore, negligence regarding the adverse drug reaction has been an area of concern globally; however, the prevalence of the issue in developing countries is higher than in developed nations. The national pharmacovigilance centre in Nigeria is responsible for regulating the country's PV activities, which is under the governance of the national agency for food and drug administration (NAFDAC). It is the responsibility of NAFDAC to protect the public from the safety hazards associated with drugs. The literature deduced that improvement in healthcare professionals' knowledge and attitude is essential for promoting efficiency in the pharmacovigilance activities in Nigeria.
Considering the rate of mortality and morbidity in Nigeria, it has been found that the high rate is due to the poor recognition of the issues brought about by the adverse drug reactions. The inattentiveness of doctors and paramedical staff towards the issue results in a high rate of hospital admissions, leading to negative economic impact and disabilities and deaths in patients. Africa is among the top consumers of pharmaceutical drugs, while the reporting of ADR cases is significantly low. This is due to a lack of sense and awareness regarding the issue among the general population as well as healthcare providers. Inappropriate medical care, use of substandard medications, limited knowledge, counterfeit products, and improper management all contribute to the increased mortality and morbidity rate. The current PV system in Nigeria is not well established to fulfil the need of the general public; therefore, the government must put in resources to provide the support needed by NAFDAC.
A significant increase in the consumption of pharmaceutical products has been witnessed in Nigeria in the last three decades; however, the government has been unsuccessful in taking steps to ensure its citizens' safety. The point of concern lies in the fact that people are consuming drugs without reporting the adverse reactions related to them. This has detrimental effects on Nigerian citizens' well-being as the government fails to detect the signal and manage them. According to the literature, in the year 2010, only 44 adverse drug reaction cases were reported in the whole year, even though Nigerians are among the actively consuming population of pharmaceutical drugs. Nigeria has four times more population than the UK, and the United Kingdom reported 86,066 cases in the same year. The comparison leaves several question marks on developing nations' strides in improving the PV system, especially in the African continent. Another reason contributing to the underreporting of ADR is the healthcare professionals' inability to extend awareness due to lack of early education. There is an utmost need to incorporate early knowledge training in the field of pharmacovigilance in Nigeria's university curriculum. The literature also shed light on a case that resulted in the death of many children in the Enugu state of Nigeria due to poorly compounded chloroquine syrup. Though NAFDAC strives to manage the ADR reporting system; however, the prevalence and usage of counterfeit drugs remain an area of concern in the country.
The survey's key findings revealed that there is a lack of awareness among the medical staff regarding the adverse drug reaction reporting system. They have no knowledge regarding the systems where they can report drug-related incidents or practice reporting ADR. The majority of the respondents have never reported an ADR. The reason for not reporting an ADR was found to include lack of knowledge about how to report, lack of or no benefit involved in reporting ADR, not enough available information to report, no idea regarding what should be reported or lack of confidence in determining if the drug caused an ADR. This signifies that healthcare professionals in Nigeria require proper training, monetary rewards and motivation to work towards improving the field of ADR reporting. The government of Nigeria must consider reporting ADR as a legal requirement for renewing the annual practising license of health care providers in the country. The practice will ensure the safe use of pharmaceutical drugs while improving pharmacovigilance practice in Nigeria subsequently.
Furthermore, it has been suggested in the research that nurses and doctors must take an active role in ensuring the safe usage of drugs and improving PV systems in Nigeria. Besides, pharmacists must also take an effective role by collaborating with doctors to use ingredients that are less likely to cause any adverse reactions. The collaboration will result in controlling the mortality rate in Nigeria. Therefore, the essential step is to monitor the increasing number of ADR cases with the help of surveillance and monitoring, which can help minimise the mortality and morbidity rate in Nigeria.
Acknowledgement
We acknowledge that we have received the approval from FCT Health Research Ethics Committee (FCT HREC), ECDA, Research ethics committee of the hospital, Kubwa General Hospital, Wuse General Hospital, Gwarinpa General Hospital, Nyanya General Hospital.
REFERENCES
- Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug safety. 2013 Feb;36(2):75-81.
- Web WH. The Importance of Pharmacovigilance-Safety Monitoring of Medicinal Products 2002. Essential Medicines and Health Products Information Portal A World Health Organization resource.
- Thobeli K. A literature review on pharmacovigilance systems in off-label use of medicines.
- Khalili H, Mohebbi N, Hendoiee N, Keshtkar AA, Dashti-Khavidaki S. Improvement of knowledge, attitude and perception of healthcare workers about ADR, a pre-and post-clinical pharmacists' interventional study. BMJ open. 2012 Jan 1;2(1).
- Gupta P, Udupa A. Adverse drug reaction reporting and pharmacovigilance: Knowledge, attitudes and perceptions amongst resident doctors. Journal of pharmaceutical sciences and research. 2011 Feb 1;3(2):1064.
- Inácio P, Cavaco A, Airaksinen M. The value of patient reporting to the pharmacovigilance system: a systematic review. British journal of clinical pharmacology. 2017 Feb;83(2):227-46.
- Agu KA, Oparah AC. Adverse drug reactions to antiretroviral therapy: Results from spontaneous reporting system in Nigeria. Perspectives in clinical research. 2013 Apr;4(2):117.
- NAFDAC. NAFDAC Good Pharmacovigilance Practice Guidelines 2016. World Health Organisation. 2016.
- Olowofela A, Fourrier-Réglat A, Isah AO. Pharmacovigilance in Nigeria: an overview. Pharmaceutical Medicine. 2016 Apr 1;30(2):87-94.
- Opadeyi AO, Fourrier-Réglat A, Isah AO. Assessment of the state of pharmacovigilance in the South-South zone of Nigeria using WHO pharmacovigilance indicators. BMC Pharmacology and Toxicology. 2018 Dec;19(1):1-8.
- Olsson S, Pal SN, Dodoo A. Pharmacovigilance in resource-limited countries. Expert review of clinical pharmacology. 2015 Jul 4;8(4):449-60.
- Chopra D, Wardhan N, Rehan HS. Knowledge, attitude and practices associated with adverse drug reaction reporting amongst doctors in a teaching hospital. International Journal of Risk & Safety in Medicine. 2011 Jan 1;23(4):227-32.
- Katusiime B, Semakula D, Lubinga SJ. Adverse drug reaction reporting among health care workers at Mulago National Referral and Teaching hospital in Uganda. African health sciences. 2015;15(4):1308-17.
- Ezeuko, Amaka Y "Adverse drug reaction reporting by different categories of healthcare workers in Nnewi, Nigeria: awareness, knowledge and attitudes." Journal of Advances in Medicine and Medical Research. 2015: 932-941
Citation: Mweetwa LL, Witika BA, Tshiamo KO, Adiukwu PC, Kenaope TJ, Oluwabusola ET et al. (2021) Investigating the Impact of Existing Pharmacovigilance Systems in Africa and How They Influence Adverse Drug Reaction Reporting by Nurses, Doctors and Pharmacists Working in Federal Government Hospitals - A Case Study of Nigeria J. Pharamacovigil. 9:314. doi-10.35248/2329-6887.21.9.314.
Copyright: © 2021 Mweetwa LL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

