Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2023) Volume 8, Issue 2
Intralesional Meglumine Antimoniate in the Treatment of Cutaneous Leishmaniasis
Georgina Heddle*Received: 16-Feb-2023, Manuscript No. JOD-23-19942; Editor assigned: 20-Feb-2023, Pre QC No. JOD-23-19942 (PQ); Reviewed: 06-Mar-2023, QC No. JOD-23-19942; Revised: 13-Mar-2023, Manuscript No. JOD-23-19942 (R); Published: 21-Mar-2023, DOI: DOI: 10.35248/2684-1436.23.8.182
Abstract
A 21 year old Pakistani male was referred to dermatology at a tertiary centre for management of cutaneous leishmaniasis. The clinical diagnosis of cutaneous leishmaniasis was confirmed by histopathology and tissue culture. There is evolving literature suggesting intralesional meglumine antimoniate is a highly efficacious and safe treatment of cutaneous leishmaniasis. We present a case of Old World cutaneous leishmaniasis which resolved with intralesional meglumine antimoniate treatment. The case highlights the potential benefits of meglumine antimoniate in the treatment of cutaneous leishmaniasis and need for prospective studies to establish the safety and efficacy of the treatment.
Keywords
Meglumine; Cutaneous Leishmaniasis; Hyperpigmentation; Erythematous; Electrocardiogram
Introduction
Cutaneous Leishmaniasis (CL) is a parasitic infestation caused by Leishmania species. There are three different clinical forms of leishmaniasis; cutaneous, mucocutaneous and visceral. The most common form of leishmaniasis is the cutaneous form. Cutaneous leishmaniasis most commonly presents as a painless erythematous papule that enlarges over weeks into an ulcerated nodule or plaque with a raised, indurated border and adherent crust of dried exudate. Currently there is no universally acceptable treatment for CL. The treatment should be individualised according to Leishmania strain and host factors. Guidelines recommend local treatment of limited and localized lesions acquired from the Old World and for localized New World CL caused by species of Leishmania that are not associated with an increased risk of mucosal disease. Systemic treatment is indicated in CL caused by species associated with high risk of mucosal disease. Intralesional Meglumine Antimoniate (MA) is widely used in Old World CL and in recent years has been approved for use in New World CL. Intralesional MA has fewer SIDE effects and lower costs compared to systemic therapy. We present a patient with CL confirmed on tissue histopathology and culture, who was managed by weekly intralesional MA with complete resolution of CL lesions after 16 weeks of treatment.
Case Report
A 21 year old male was referred to dermatology at a tertiary centre for management of Cutaneous Leishmaniasis (CL). Besides CL, the patient denied prior medical history and denied regular medications. The patient had migrated from Pakistan to Australia at thirteen years of age. An erythematous papular rash developed on the patient’s right cheek following a sandfly bite to the area when he was seven years old. Over the following years, the rash became a larger confluent plaque. The diagnosis of CL was made in Pakistan however, the patient was unable to recall how the diagnosis was made. The plaque was treated with multiple topical, intralesional and intramuscular therapies in Pakistan, however the patient and his family were unable to recall the specific treatments he received. In 2015, the patient presented to a dermatology service in Australia with persistence of the right cheek plaque. Histopathology of the right cheek demonstrated confluent non-necrotising granulomas, intervening lymphocytic infiltrate and Langhans-type giant cells with nuclei in a horse-shoe pattern, as demonstrated in Figure 1.
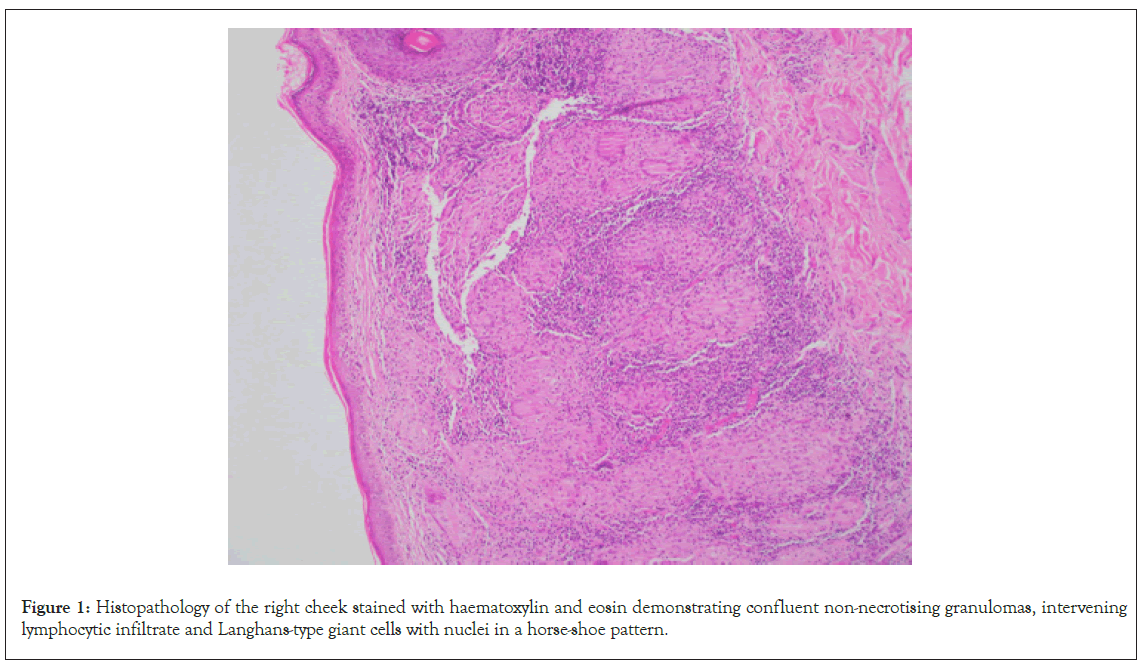
Figure 1: Histopathology of the right cheek stained with haematoxylin and eosin demonstrating confluent non-necrotising granulomas, intervening lymphocytic infiltrate and Langhans-type giant cells with nuclei in a horse-shoe pattern.
The patient went on to receive multiple different treatments. In 2016, topical paromomycin was used for 3 months but was ineffective and subsequently ceased. Oral itraconazole was then used for 2 months without response. The patient reported flattening of the plaque in 2017 following 4 weeks of weekly intalesional stibogluconate. Whilst awaiting the approval of stibogluconate application in 2018, the plaque worsened despite treatment with cryotherapy. The patient was lost to follow up after he moved overseas and subsequently the papules slowly returned.
On presentation to dermatology in 2022, there was a pink plaque on the right cheek with seven fleshy papules, varying from 2 mm to 10 mm, on a background of mild erythema, dyspigmentation and atrophic scarring (Figure 2). Treatment with intralesional stibogluconate was considered initially, given past effectiveness for this patient. However, access to stibogluconate in Australia proved difficult due to importation restrictions. Systemic antimonials were not chosen due to potentially significant side effects and the need for hospital admission for electrocardiogram and blood monitoring. Ultimately the decision was made to treat the plaques with intralesional Meglumine Antimoniate (MA) based on literature demonstrating good efficacy and safety of MA in clinically simple, localised and limited CL lesions. Initially 0.5-1 mL was injected into each nodule once weekly, with a maximum dosing of 5 mL injected per session. After a few weeks, the dose was increased to 3.0-4.5 mL per nodule. A more rapid reduction in the size of the nodules and erythema was observed with higher MA dosing. The patient received a total of 16 intralesional MA injections over 16 weeks with an excellent response and only postinflammatory hyperpigmentation remaining. Improvement of the CL lesions during treatment is demonstrated in Figure 3.
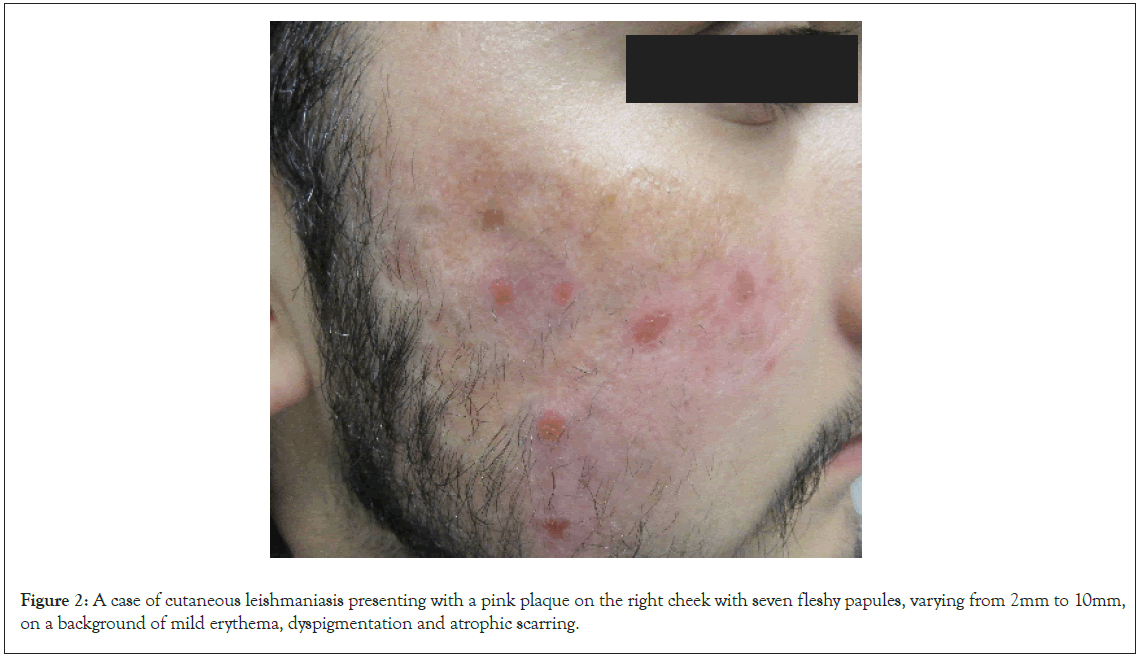
Figure 2: A case of cutaneous leishmaniasis presenting with a pink plaque on the right cheek with seven fleshy papules, varying from 2mm to 10mm, on a background of mild erythema, dyspigmentation and atrophic scarring.
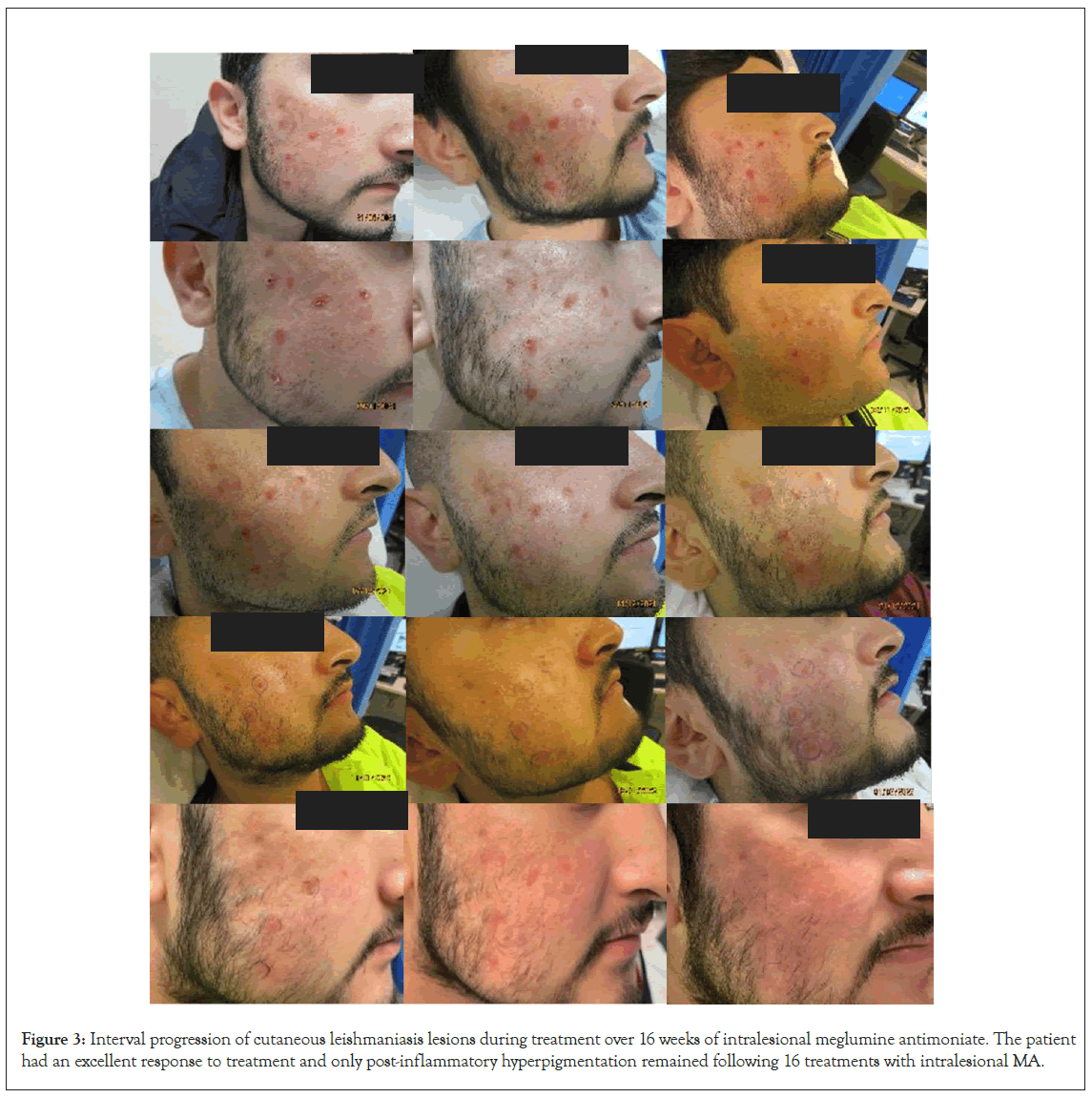
Figure 3: Interval progression of cutaneous leishmaniasis lesions during treatment over 16 weeks of intralesional meglumine antimoniate. The patient had an excellent response to treatment and only post-inflammatory hyperpigmentation remained following 16 treatments with intralesional MA.
Discussion
Leishmaniasis is a chronic vector-borne infection caused by numerous species of Leishmania protozoa. Transmission of the disease occurs through the bite of an infected female sand fly [1]. The disease is traditionally classified as either ‘Old World’ or ‘New World’ leishmaniasis. Old world Leishmaniasis is widespread in the Middle East, Mediterranean, Africa and Asia and is most commonly caused by L. major, L. tropica, L. aethiopica and some zymodemes of L. infantum [2]. Leishmania acquired in the ‘New World’, consisting of America, Central America, Mexico and South America, are most commonly associated with L. braziliensis and L. Mexicana and related species [3].
Leishmaniasis can manifest in three different clinical forms: Cutaneous Leishmaniasis (CL), Mucocutaneous Leishmaniasis (ML) and Visceral Leishmaniasis (VL). Cutaneous leishmaniasis is the most common form globally. Cutaneous lesions of CL include ulcers, nodules or plaques and typically affect exposed regions of the body, mainly the face, arms and legs. Most commonly CL presents as a painless erythematous papule that enlarges over weeks into an ulcerated nodule or plaque with a raised, indurated border and adherent crust of dried exudate. Ulcerated and crusted nodules and plaques have a tendency to develop along the skin folds. There are multiple variants of cutaneous leishmaniasis, including eczematous, psoriasiform, varicelliform, zosteriform, paronychial, annular, whitlow-like, verrucous, keloidal, chanciform and erysipeloid variants [4]. Secondary bacterial infection is common and can result in pain [5]. Some ‘New World’ strains of Leishmaniasis, including L. (Viannia) braziliensis and related species in the Viannia subgenus, are associated with increased risk of mucosal leishmaniasis (ML). Mucosal leishmaniasis can result in destructive naso-oropharyngeal/laryngeal mucosal lesions.
Diagnosis of CL can be made through culture of lesional skin scrapings and histopathological studies of tissue section from the border of an active cutaneous lesion. Parasites in free form or within macrophages may be seen on smear, or less often within polymorphonuclear leukocytes. Tissue sections with haematoxylin and eosin staining demonstrate epidermal atrophy or hyperplasia. Inflammatory infiltrate consisting of macrophages, lymphocytes and plasma cells is present with regions of focal necrosis. Early in the infection, Leishmania protozoa may be seen inside cytoplasmic vacuoles in histiocytes, known as Leishman bodies. Infected macrophages are less frequent in late stages of the infection. Few amastigotes and some lympho-histiocytic infiltrates may be present confirming a tuberculoid granuloma in this later phase. The parasites can be seen on smear with Giemsa or Wright stain. Identification of the causative Leishmania species is important for clinical management decisions.
There is no universally applicable treatment for CL to date. The treating agent, dose and duration should be individualised according to Leishmania strain and host factors, for example immunologic status and comorbidities. The Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH) were developed to provide guidance on the diagnosis and management of Leishmaniasis. The guidelines suggest that risk of ML and other complications are low in immunocompetent patients if the infection is caused by a subgenus other than the Viannia species and the lesions are clinically simple, as defined in Table 1, and benign or healing spontaneously. In these patients with low risk of ML, observation is reasonable. Subsequent treatment may be provided to patients whose lesions do not heal. The main concern regarding management of CL with observation only is the risk of metastatic infection to the mucosa causing destructive lesions that are difficult to treat and potentially leading to severe sequelae. Therefore, regular follow up with careful nasooropharyngeal examination is recommended during observation.
| Simple CL | Complex CL |
|---|---|
| Caused by Leishmania species unlikely to be associated with Mucosal Leishmaniasis (ML) | Caused by a Leishmania species that can be associated with increased risk for ML, particularly Viannia spp. in the "mucosal belt" of Bolivia, Peru and Brazil |
| No mucosal involvement | Local subcutaneous nodules |
| Absence of characteristics of complex CL | Large regional adenopathy |
| Only a single or a few skin lesions | >4 skin lesions of substanital size (>1cm) |
| Small lesion size (diameter <1cm) | Large individual skin lesion (diameter >5cm) |
| Location of lesion feasible for treatment | Size or location of lesion such that local treatment is not feasible |
| Nonexposed skin (ie. cosmetically not important) | Lesion on face, including ears, eyelids, or lips; fingers, toes, or other joints; or genitalia |
| Immunocompetent host | Immunocompromised host |
| Lesion(s) resolving without prior therapy | Clinical failure of local therapy |
| - | Unusual syndromes: leishmaniasis recidivans, diffuse CL, or disseminated CL |
Table 1: Clinical characteristics of Cutaneous Leishmaniasis (CL) that may modify management.
Generally, local treatment is preferred for treatment of localised and limited lesions, defined as clinically simple, acquired from the Old World and for localized New World CL caused by species of Leishmania that are not associated with an increased risk of mucosal disease. Local treatments include topical paromomycin preparations, intralesional injection of pentavalent antimonial drugs (including sodium stibogluconate and MA), cryotherapy, photodynamic or laser therapy. The (Institute for Defence Studies and Analysis) IDSA and American Society of Tropical Medicine Hygiene (ASTMH) guidelines recommend debridement of overlying eschar prior to administration of local therapy to maximise treatment efficacy. Oral or parental systemic therapy can be considered in patients who do not respond to initial local therapy. Local treatment is generally not recommended for CL with high risk of mucosal dissemination.
Systemic treatment is recommended in patients with complex CL, as defined in Table 1. CL caused by species associated with high risk of ML or if the Leishmania species is unknown or when the infection was acquired in a region with increased ML-risk, systemic treatment is indicated. The current guidelines recommend systemic treatments for these patients even if lesions are healing or have recently healed. Rarer cutaneous syndromes, including leishmaniasis recidivans (caused by L. tropica and occasionally other species), diffuse cutaneous leishmaniasis (caused by L. Mexicana, L. amazonesis and L. aethiopica) and disseminated cutaneous leishmaniasis (caused by L. braziliensis) typically require systemic therapy. Systemic treatments include amphotericin B deoxycholate or lipid formulations, pentavalent antimicrobial derivatives (for example, sodium stibogluconate and MA), pentamidine, miltefosine and azole antifungals.
Sodium stibogluconate is commonly used to treat New World CL in America with greater than 90% of cases achieving complete resolution with a single 20 day course [6-9]. However toxicity is common with systemic sodium stibogluconate with most patients experiencing malaise, anorexia, myalgia and arthralgia following 14 days of treatment. Other associated side effects include elevation of serum aminotransferases, leukopenia, thrombocytopenia, pancreatitis and Electrocardiogram (ECG) changes and cardiotoxicity.
In the case described, the CL lesions were treated with intralesional MA with resolution of lesions. Intralesional MA is widely used in Old World CL and in recent years has been approved for use in New World CL. Potential side effects of MA include localised pruritis, oedema, pain and erythema and infection. Compared to systemic therapy, intralesional MA has fewer side effects and lower costs. There are several case series demonstrating good safety and efficacy of intralesional MA for CL. Intralesional MA for localised CL was studied in a single-arm phase II clinical trial in Brazil. A definitive cure rate of 87% was achieved at day 180 in 53 patients (62 lesions) receiving a median of seven intralesional injections over a median treatment period of 43 days. Most adverse effects in the trial were local and mild to moderate in severity. In a case series of 12 patients receiving intralesional MA for New World CL, 11 patients (91.6%) achieved complete cure and only one patient did not respond to treatment. These findings are comparable to a prospective study by Calvopina et al. of 21 patients with CL treated with intralesional MA. After 30 days of treatment, 19 patients (90.5%) achieved complete resolution of their lesions.
Conclusion
The case highlights the potential benefits of treatment of Cutaneous Leishmaniasis (CL) with intralesional Meglumine Antimoniate (MA). Whilst current literature suggests that intralesional MA is likely a safe and effective treatment of localised and limited CL, studies are currently limited by small sample sizes. Further prospective studies with larger samples are needed to establish the true efficacy and safety of intralesional MA for CL.
References
- De Vries HJ, Schallig HD. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. Am J Clin Dermatol. 2022;23(6):823-840.
[Crossref] [Google Scholar] [PubMed]
- Weina PJ, Neafie RC, Wortmann G, Polhemus M, Aronson NE, Strausbaugh LJ. Old world leishmaniasis: an emerging infection among deployed US military and civilian workers. Clin Infect Dis. 2004;39(11):1674-1680.
[Crossref] [Google Scholar] [PubMed]
- Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63(12):202-264.
[Crossref] [Google Scholar] [PubMed]
- Murray HW. Leishmaniasis in the United States: treatment in 2012. Am J Trop Med. 2012;86(3):434.
[Crossref] [Google Scholar] [PubMed]
- Grayson W, Calonje E. Infectious disease of the skin. McKee’s Pathology of the Skin, 5th edn. Cleveland: Elsevier, 2020;18:904-912.
- Ramalho DB, Silva RE, Senna MC, Moreira HS, Pedras MJ, Avelar DM, et al. Meglumine antimoniate intralesional infiltration for localised cutaneous leishmaniasis: a single arm, open label, phase II clinical trial. Mem Inst Oswaldo Cruz. 2018;113.
[Crossref] [Google Scholar] [PubMed]
- Brito NC, Rabello A, Cota GF. Efficacy of pentavalent antimoniate intralesional infiltration therapy for cutaneous leishmaniasis: A systematic review. PloS one. 2017;12(9):e0184777.
[Crossref] [Google Scholar] [PubMed]
- Arboleda M, Barrantes S, Usuga L, Robledo S. Successful treatment of cutaneous leishmaniasis with intralesional meglumine antimoniate: A case series. Rev Soc Bras Med Trop. 2019;52;e.20180211.
- Calvopiña M, Cevallos W, Paredes Y, Puebla E, Flores J, Loor R, et al. Intralesional infiltration with meglumine antimoniate for the treatment of leishmaniasis recidiva cutis in Ecuador. Am J Trop Med. 2017;97(5):1508.
[Crossref] [Google Scholar] [PubMed]
Citation: Heddle G (2023) Intralesional Meglumine Antimoniate in the Treatment of Cutaneous Leishmaniasis. J Dermatitis. 8:182
Copyright: © 2023 Heddle G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.