Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 5
Influence of Novel Coronavirus COVID-19 and HIV: A Scoping Review of Hospital Course and Symptomatology
Mona Sheikh1,3*, Shavy Nagpal2*, Madiha Zaidi1, Rupalakshmi Vijayan1, Wanessa Matos1, Neguemadji Ngardig Ngaba1, Lordstrong Akano1, Samia Jahan1, Shazia Q. Shah1, Camille Celeste Go4, Sindhu Thevuthasan1 and George Michel52Department of Surgery, The Research Institute of St. Joe's Hamilton, ON, Canada
3University of North Texas Health Science Center, Fort Worth,Texas, USA
4Department of Family Medicine, Larkin Community Hospital Palm Springs Campus, Florida, USA
5Department of Internal Medicine, Larkin Community Hospital South Miami, Florida, USA
Received: 02-Aug-2021 Published: 23-Aug-2021, DOI: 10.35248/2157-7560.21.12.461
Abstract
Background: An outbreak of novel coronavirus (SARS-CoV-2) was observed on December 2019 in Wuhan, China which led to a global pandemic declared in March 2020. As a consequence, it imposed delirious consequences in patients with underlying co-morbid conditions that make them immunocompromised. The purpose of this paper is to provide an in depth review of influence of COVID-19 in patients with underlying HIV in terms of mortality and hospitalization.
Authors also aim to provide a thorough risk analysis of hospitalization, ICU admission and mortality of PLWH and COVID-19. The secondary objective was to analyze the CD4+ count variations and outcome of COVID-19 and to correlate if ART provided a protective role. Authors also aim to provide an evaluation of typical clinical presentation of COVID-19 in PLWH. ART is found to show activity against SARS-CoV-2 in vitro, and there is some similarity in the structure of HIV-1 gp41 and S2 proteins of SARS-CoV since they both belong to +ssRNA type.
Methods: We conducted a literature review using search engines namely, Cochrane, PubMed and Google Scholar. The following keywords were targeted: “COVID-19,” “SARS-CoV-2,” and “HIV.” We included case reports, case series, and cohort (retrospective and prospective) studies. We excluded clinical trials and review articles. We came across 23 articles that met the inclusion criteria. PRISMA guidelines were followed for study acquisition.
Results: From the 23 studies, we found a total of 651 PLWH with confirmed COVID-19 (549, 91, and 11 in cohorts, case series, and case reports, respectively). The overall risk of hospital admission from pooled data of the 23 reviewed articles was 69.13% (450/651), ICU admission was 12.90% (84/651) in total infected patients, and 18.67% (84/450) among hospitalized patients. The overall case fatality rate from the 23 reviewed articles was 11.21 (73/651).A weak positive correlation was found between CD4+ counts and hospital admissions in case series and case reports, while the weak negative correlation was found in cohorts. For mortality, there was a negative weak association in the cohorts and in case series, while a weak positive was seen in case reports. We assessed the presenting symptoms of PLWH with COVID-19, and our review demonstrated this group does not greatly differ from the rest of the population, as their common presenting symptoms were cough, fever, and SOB.
Conclusion: Our results indicated that there was a high rate of hospitalization, ICU admission, and mortality among patients Living with HIV and COVID-19. PLWH needs to be noted as a high-risk group for COVID-19 complications and severity. We recommend that PLWH be closely monitored by their physicians and strictly adhere to antiretroviral therapy and standard universal COVID-19 precautions.
Keywords
HIV; COVID-19; Mortality; SARS-CoV-2; Coronavirus
Abbreviations
HIV: Human Immunodeficiency Virus; ART: Antiretroviral Therapy; ICU: Intensive Care Unit; PLWH: People living with HIV; +SSRNA: Positive Single Stranded RNA; SOB: Shortness of Breath; HTN: Hypertension; DM: Diabetes Mellitus; CKD: Chronic Kidney Disease; COPD: Chronic Obstructive Pulmonary Disease; CVD: Cardiovascular Disease; HLD: Hyperlipidemia; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus, CAD: Coronary Artery Disease; ESRD: End Stage Renal Disease; NASH: Non-Alcoholic Steatohepatitis; OSA: Obstructive Sleep Apnea; CHF: Congestive Heart Failure; TB: Tuberculosis.
Introduction
SARS-CoV-2 is a member of β-CoVs (Figure 1) and is a global concern at present. What interests Authors were the overlapping similarities between the Human Immunodeficiency Virus (HIV) and SARS-COV-2 virus in terms of same RNA single-stranded family and protein structural motifs of HIV-1 gp41 and SARSCoV- S2 [1-9]. Having said that it was proposed in clinical trials to implement ART as a treatment modality for COVID-19, however, it is not recommended by the Treatment Guidelines Panel for COVID-19 yet [10].
The prognosis of COVID-19 in common comorbidities such as HTN, DM and CVD is well known but its prognosis or outcome of patients living with HIV is not well understood so far [11]. This review article focuses on the people living with HIV which are confirmed COVID-19 positive cases.
The main focus of our review is to assess the risk of hospitalization, intensive care unit (ICU) admission, invasive mechanical ventilation (IMV) requirement and mortality in persons living with HIV (PLWH) who were COVID-19 positive. The secondary objective is to assess whether any correlation exists between CD4+ count and COVID-19 in PLWH with the third objective to evaluate the typical clinical presentation of COVID-19 in such patients.
Methodology
Literature search
A literature search was conducted with PubMed, Cochrane, and Google scholars using the following keywords: “COVID-19”, “HIV,” “SARS-CoV-2”, and “AIDS” from January 2020 until August 31st, 2020. Only papers in English language were considered. PRISMA guidelines were followed (Figure 2).
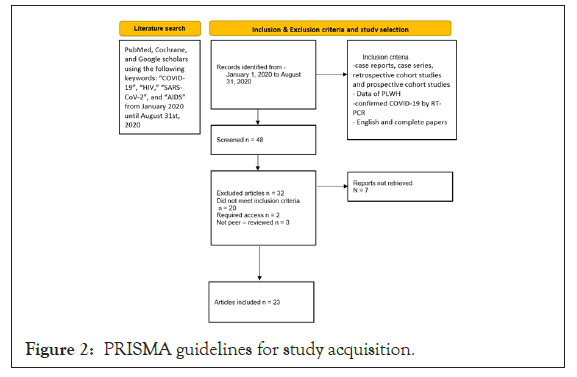
Figure 2: PRISMA guidelines for study acquisition.
Inclusion and exclusion criteria and study selection
The case reports, case series, retrospective cohort studies and prospective cohort studies published between January 1, 2020, and August 31, 2020 with data of PLWH and confirmed COVID-19 by RT-PCR were included in our review. Review articles, clinical trials, and preprint articles were excluded. Articles that did not have patient data and those limited to specific comorbidities and organ dysfunctions were also excluded to avoid selection bias.
We narrowed it down to 48 articles that were screened based on the relevancy of the titles and abstracts. We excluded 32 articles, of which 20 did not meet the inclusion criteria, 2 required access, and 3 were not peer-reviewed. We included 23 studies in this paper. Selected articles were independently reviewed by two authors. All disagreements were resolved with a discussion between the two authors or with input from a third independent reviewer and mutually agreed upon by the authors.
Data extraction
The authors designed a predefined data extraction list to be used to extract the required data from the included studies. The following data were extracted: First author’s name, publication date, study design, country, the total number of PLWH with confirmed COVID-19 by RT-PCR or antigen test, age, gender, comorbidities, ART, CD4+ cell counts, viral load, number of hospitalized patients (age, gender), duration of hospitalization, number admitted to ICU, a number that needed IMV and number of deaths (age, gender, comorbidity, CD4+ counts, ART list).
Data collection and analysis
The data was collected on excel sheets and Google documents. We ran a qualitative review based on our primary and secondary outcomes. The studies used have been listed in Tables1-3. The data were tabulated using Microsoft Excel.
The Pearson correlation coefficient was used to assess the correlation between CD4+ counts and hospitalization and mortality, and the association of COVID-19 infection with hospital admission. A P-value <0.05 was considered significant and was calculated by using R factor, Z score and standard deviation.
Ethical approval and funding
We did not seek IRB approval, as our study is based on data from published articles and patients were not directly involved. No funding was obtained for this review.
Results
This review included a total of 23 articles after excluding those that did not meet the inclusion criteria, not peer reviewed, and required access. The 23 articles were 7 cohort studies, 7 case series, and 9 case reports; (Figure 3) and they were from different countries: USA, China, Spain, Italy, the UK, Germany, Turkey, India, Japan and Uganda [1-9] (Figure 4).
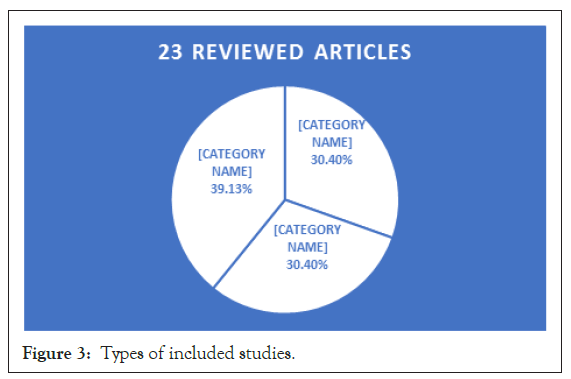
Figure 3: Types of included studies.
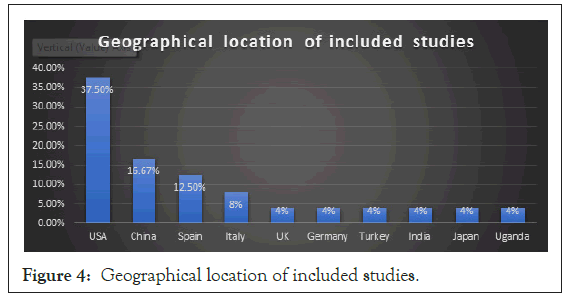
Figure 4: Geographical location of included studies.
Our review was categorized into three groups based on the similarity of study type: which are Cohort group (Ch), Case Series group (CS), and Case Report group (CR). The number of PLWH and confirmed COVID-19 were 549 from cohort studies, 91 from case series, and 11 from Case Reports (CR). The mean ages (in years) were 54 in Ch, 45 in CS, and 36 in CR. The gender distribution in the Ch was 440 male, 106 female, and 3 transgender; in CS was 62 male, 25 female and 3 transgender; and in CR was 8 males and 3 females (Tables 1-3).
| Author/Study type | Location | Total no. infect. pts | Total no. hosp. pts | Rate of hosp. | Total no. ICU pt. | ICU rate (total pt., hosp.) | Total no. IMV pt. | Rate of IMV (total pt., hosp., ICU) | Total deaths | Mortality Rate (total infect) |
|---|---|---|---|---|---|---|---|---|---|---|
| Viscarra et al./ Prospective | Spain | 35 | 28 | 80% | 6 | 21.4%, 17.1% | 5 | 14.3%, 17.9% | 2 | 5.70% |
| Del Amo et al./Prospective | Spain | 236 | 151 | 64% | 15 | 9.9%, 6.4% | NA | N/A | 20 | 8.50% |
| Ho et al./ Retrospective | USA | 93 | 72 | 77.40% | 19 | 20.4%, 26.4% | 15 | 16.1%, 20.8% | 19 | 20.40% |
| Sigel et al. / Retrospective | USA | 88 | 88 | 100% | 18 | 20.5%, 205% | 18 | 20.5%, 20.5% | 18 | 20.50% |
| Meyerowitz et al. / Retrospective | USA | 36 | 21 | 58% | 6 | 28.6%, 16.6% | 4 | 11.1%, 19.1% | 2 | 5.60% |
| Gervasoni et al. / Retrospective | Italy | 28 | 13 | 50% | 2 | 15.4%, 7.1% | 2 | 4.3%, 15.4% | 2 | 4.30% |
| Harter et al. / Retrospective | Germany | 33 | 14 | 42% | 6 | 42.9%, 18.2% | 4 | 12.1%, 28.6% | 3 | 9.10% |
| Total | 549 | 387 | 70.49% | 72 | 13.11%, 18.61% | 48 | 8.74%, 12.70%, 66.67% | 66 | 12.02% |
Table 1: Outcome of PLWH with COVID-19: Cohort studies.
| Author/Study type | Location | Total no. infect pts. | Total no. hosp. pts | Rate of hosp | Total no. ICU pts | ICU rate (total pt., hosp.) | Total no. IMV pts. | Rate of IMV (total pt., hosp., ICU) | Total deaths | Mortality Rate (total infected) |
|---|---|---|---|---|---|---|---|---|---|---|
| Blanco et al. / Case Series | Spain | 5 | 5 | 100% | 2 | 40%, 40% | 1 | 20%, 20% | 0 | 0 |
| Childs et al. / Case Series | USA | 18 | 18 | 100% | 5 | 27.7%, 27.7% | 5 | 27.78%, 27.8% | 5 | 27.80% |
| Ridgeway et al. / Case Series | USA | 5 | 5 | 100% | 1 | 20%, 20% | 0 | 0 | 0 | 0 |
| Byrd et al. / Case Series | USA | 27 | 9 | 33.30% | 0 | 0 | 0 | 0 | 1 | 3.70% |
| Altuntas Aydin et al. / Case Series | Turkey | 4 | 4 | 100% | 1 | 25%, 25% | 1 | 25%, 25% | 0 | 0 |
| Marimuthu et al. / Case Series | India | 6 | 6 | 100% | 0 | 0 | 0 | 0 | 0 | 0 |
| Calza et al. / Case Series | Italy | 26 | 5 | 19.20% | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 91 | 52 | 57.14% | 9 | 9.89%, 17.31% | 7 | 7.69%, 13.46%, 77.78% | 6 | 6.59% |
Table 2: Outcome of PLWH with COVID-19: Case series.
| Author/Study type | Location | Total no. infected pts | Total no. hosp. pts | Rate of hosp. | Total no. ICU pt. | ICU rate (total pt., hosp.) | Total no. IMV pt. | Rate of IMV (total pt., hosp., ICU) |
|---|---|---|---|---|---|---|---|---|
| Zhu et al. / Case Report | China | 1 | 1 | 100% | 0 | 0 | 0 | 0 |
| Zhao et al. / Case Report | USA | 1 | 1 | 100% | 0 | 0 | 0 | 0 |
| Wang et al. / Case Report | China | 1 | 1 | 100% | 1 | 100%, 100% | 0 | 0 |
| Kiran Rashed et al. / Case Report | USA | 1 | 1 | 100% | 0 | 0 | 0 | 0 |
| Toombs et al. / Case Report | UK | 3 | 3 | 100% | 1 | 33.3%, 33.3% | 1 | 33.3%, 33.3%, 100% |
| Baluku et al. / Case Report | Uganda | 1 | 1 | 100% | 0 | 0 | 0 | 0 |
| Nakamoto et al. / Case Report | Japan | 1 | 1 | 100% | 0 | 0 | 0 | 0 |
| Hadded et al./ Case Report | USA | 1 | 1 | 100% | 1 | 100%, 100% | 1 | 100%, 100% |
| Menghua et al. / Case Report | China | 1 | 1 | 100% | 0 | 0 | 0 | 0 |
| Total | 11 | 11 | 100% | 3 | 27.27% both | 2 | 18.18%, 18.18%, 66.67% |
Table 3: Outcome of PLWH with COVID-19: Case reports.
Risk of hospitalization of PLWH+ confirmed COVID-19
The total numbers of hospitalized patients were 387 in Ch, 52 in CS, and 11 in CR. The risk of hospitalization in these 3 groups were 70.49% (387/549) in Ch, 57.14% (52/91) in CS, and 100% in CR (Tables 1-3). The overall risk of hospital admission from pooled data of the 23 reviewed articles was 69.13% (450/651).
Risk of ICU admission among hospitalized patients
The number of patients that needed critical care was 72 in Ch, 9 in CS, and 3 in CR. The calculated risk among hospitalized patients was 18.61% (72/387) in Ch, 17.31% (9/52), and 27.27% (3/11) in CR (Tables 1-3). The overall risk of ICU admission from pooled data of the 23 reviewed articles was 12.90% (84/651) in total infected patients and 18.67% (84/450) among hospitalized patients.
The risk of IMV requirement in hospitalized and ICU patients
Those patients who were admitted to the ICU: 48 in Ch, 9 in CS, and 2 patients in CR needed invasive mechanical ventilation (IMV). The risk among hospitalized and ICU patients was (12.7%, 66.67%) in Ch, (13.46%, 77.78%) in CS, and (18.18%, 66.67%) in CR (Tables 1-3).
The mortality rate of PLHW+ confirmed COVID-19
The total number of deaths in the three categories was 66 in Ch, 6 in CS, and 1 CR, respectively. The case fatality rate was 12.02% (66/549) in Ch, 6.59% (6/91) in CS, and 9.09% (1/11) in CR (Tables 1-3). The overall case fatality rate from the 23 reviewed articles was 11.21 (73/651).
Correlation of CD+ counts with hospitalization and mortality
The Pearson Correlation coefficient was used to assess the association of CD4+ counts with hospitalization and mortality in PLWH+COVID-19. A weak positive correlation was found between CD4+ counts and hospital admissions in case series (r=0.2622, p-value=0.089403) (Figure 5) and case reports (r=0.2269, p-value=0.5284) (Figure 6), while the weak negative correlation was found in cohorts (r=-0.114, p-value=0.043866) (Figure 7). For the mortality correlation, there was a negative weak association in the cohorts (r=-0.1218, p-value=0.031) (Figure 8) and in case series (r=- 0.011, p-value=0.986) (Figure 9), while a weak positive was seen in case reports (r=0.0667) (Figure 10).
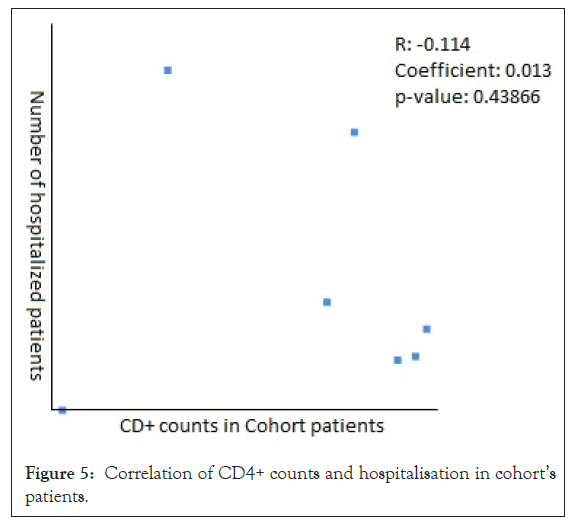
Figure 5: Correlation of CD4+ counts and hospitalisation in cohort’s patients.
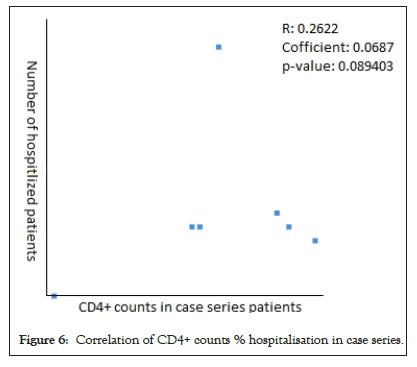
Figure 6: Correlation of CD4+ counts % hospitalisation in case series.
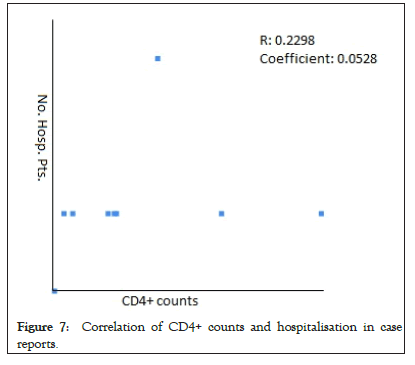
Figure 7: Correlation of CD4+ counts and hospitalisation in case reports.
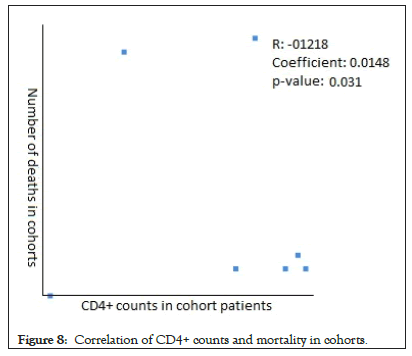
Figure 8: Correlation of CD4+ counts and mortality in cohorts.
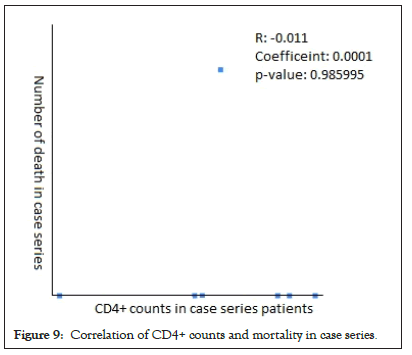
Figure 9: Correlation of CD4+ counts and mortality in case series.
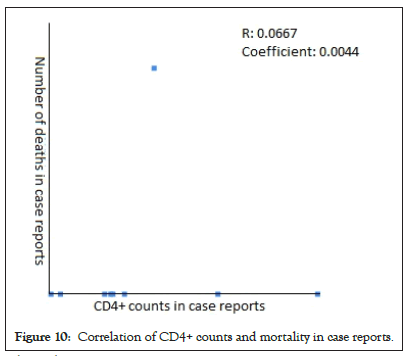
Figure 10: Correlation of CD4+ counts and mortality in case reports.
Clinical presentations in PLWH+ COVID-19
The clinical presentation from the pooled data of the 23 articles was available for 319 patients (Figure 11). The most common symptoms among these patients were cough (57.4%, no=183), fever (55.5%, no=177), SOB (21.9%, no=70), myalgia (13.2%, no=42), fatigue (11.9%, no=38), and headache/malaise (11%, no=35). And least common with less than 10% were dyspnea (9.7%, no=31), gastrointestinal symptoms (nausea/vomiting/diarrhea), (6.6%, no=21), sore throat (6.3%, no=20), anosmia/ageusia (4.4%, no=14), and altered mental status (4.4%, no=14) (Table 4).
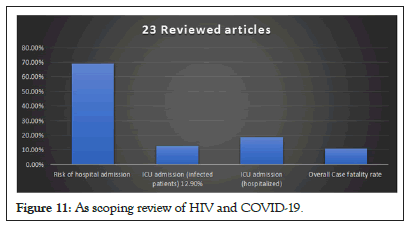
Figure 11: As scoping review of HIV and COVID-19.
| Author (no. death) | Age (mean) Gender |
Comorbidities | ART | CD4+ counts |
|---|---|---|---|---|
| Viscarr et al. (no=2) | N/A | N/A | N/A | 1st pt.: 137 2nd pt.: 636 |
| Del Amo et al. (no=20) | N/A 16 m, 4 f |
N/A | -10 pt. on TAF/FTC -8 pt. on ABC/3TC |
N/A |
| Ho et al. (no=19) | 62 (55-68) 13 m, 6 f |
13 obesity | -10 pt. on TDF -3 pt. on PI |
686 (466-800) |
| Sigel et al. (no=18) | 62 (57-67) 13 m, 5 f |
4 DM, 6 HTN, 2 COPD, 6 CKD, 3 organ transplant, 1 liver cirrhosis, 1 obesity | -13 pt. on Integrase -5 pt. on PI -16 pt. on NRT |
1 pt. < 50 6 pt. 50-200 6 p.t 201-500 1 pt. >500 |
| Meyerowitz et al. (no=2) | 59, 63 1 m, 1 f |
1st pt.: CHF, CKD, DM, HLD, HTN, prior stroke, 40 BMI 2nd pt.: HTN, prior stroke, CKD |
-1st pt.: CTG+ABC/ETG+DRT/r+ETR -2nd pt.: DTG+TAF/FTC |
426 |
| Gervasoni et al. (no=2) | 47, N/A 2 m |
-1st pt.: obesity -2nd pt.: CVD, lung cancer |
N/A | N/A |
| Harter et al. (no=3) | 59, 55, 82 3 m |
-1 pt.: HTN, COPD, DM -other 2: N/A |
-1st pt.: DOR/TDF/FTC -2nd pt.: BIC/TAF/FTC -3rd pt.: DRV/RTV/RGV |
388.7 (718, 69, 379) |
| Byrd et al. (no=1) | 59 m |
ESRD, cancer | EFV, ABC | 936 |
Table 4: Outcome of PLWH with COVID-19: Case reports.
Co-morbidities in COVID positive PLWH groups
The comorbidities from the 23 articles were reported for 417 out of 649 patients; of which the most common chronic medical conditions found were hypertension (37.4%, no=156), diabetes mellitus (21.8%, no=91), chronic kidney disease (12.2%, no=51), COPD/asthma/lung disease (12.2%, no=51), cancer (8.9%, no=37), obesity (6.7%, no=28), cardiovascular disease (6.2%,no=26), chronic liver disease (5.5%, no=23), and hyperlipidemia (5.8%, no=24) (Table 5).
| Author (no= T. PLWH + COVID) | Comorbidities | Clinical Presentation | Viral Load | CD4+ counts | ART, Y or N/% | T. hosp. | T. deaths |
|---|---|---|---|---|---|---|---|
| Viscarra et al. (no=5) | 15 (42.9%) HTN, 10 %) CVD, 6 (17.1%) DM, 3 (8.6%) CKD, 16 (45.7%)chronic liver disease, 8 (22.9%)chronic respiratory disease, 9 (25.7%) cancer | 25 fever, 7 sore throat, 24 cough, 21 dyspnea, 4 anosmia/ageusia, 13 myalgia, 8 headache, 21 fatigue, 10 diarrhea, 3 N/V | 34 pt.: <50 | 502 (295 – 771) | Y/100% | 28 | 2 |
| Del Amo et al. (no=236) | N/A | N/A | N/A | N/A | 100% | 151 | 20 |
| Ho et al. (no=93) | 49 (52.7%) HTN, 32 (34.4%) DM, 25 (26.8%) COPD/Asthma, 16 (17.2%) CKD, 8 (8.6%) cancer, 7 (7.5%) ESRD, 4 (4.3%) autoimmune disease | 61 fever, 71 cough, 57 SOB, 10 altered mental status | 57 pt.: <50 | 554 (339-752) | Y/95.7% | 72 | 19 |
| Sigel et al. (no=88) | 33 (37.5%) HTN, 24 (27.3%) DM, 5 (5.7%) liver cirrhosis, 6 (6.8%) CAD, 19 (21.6%) CKD, 15 (17.1%) cancer, 4 (4.6%) organ transplant, 9 (10.2%) obesity, 48 (54.6%) smoking | N/A | -66 pt.: <50 | <50->500 | Y/100% | 88 | 18 |
| -16 pt.: >50 | |||||||
| Meyerowitz et al. (no=36) | 11 (30.6%) HTN, 8 (22.2%) DM, 4 ( 11.1% CHF, 8 (22.2%) HLD, 6 (16.7%) CKD, 2 (5.6%) ESRD, 5 (13.9%) stroke, 3 (8.3%) COPD, 3 (8.3%) smoking, 1 (2.8%) NASH, 1 (2.8%) NAFLD, 1 (2.8%) asthma, 1 (2.8%) dementia, 2 (5.6%) lymphomas (in remission) | 22 fever, 21 cough, 6 sore throat, 8 myalgia, 5 fatigue, 3 dyspnea, 13 SOB, 10 myalgia, 4 headache, 3 altered mental status, 4 anosmia/ageusia, 7 GI (diarrhea, appetite loss, N/V),3 congestion | N/A | 691 (16 – 1485) | Y/97.2% | 21 | 2 |
| Gervasoni et al. (no=28) | 14 (50%) HTN, 3 (10.7%) DM, 15 (53.6%) HLD, 5 (17.9%) HCV/HBV co-infection, 4 (14.3%) renal disease, 2 (7.1%) epilepsy, 2 (7.1%) CVD, 3 (10.7%) neoplasm, 2 (7.1%) COPD, 1 (3.6%) organ transplant | 41 fever, 23 cough, 10 dyspnea, 7 diarrhea, 4 myalgia, 3 headaches | <50 | 636 (346 – 926) | Y/100% | 13 | 2 |
| Harter et al. (no=33) | 10 (30.3%) HTN, 4 (12.1%) DM, 6 (18.2%) COPD, 2 (6.1%) CVD, 2 (6.1%) renal disease, 5 (15.2%) HBV, 3 (9.1%) CVD, 1 (3%) HCV | 25 cough, 22 fever, 7 myalgia/arthralgia, 7 headache, 7 sore throat, 6 anosmia | -30 pt.: <50 | 670 (69-1715) | Y/100% | 14 | 3 |
| -920 | |||||||
| -824 | |||||||
| Blanco et al. (no=5) | 1 (20%) hypothyroidism, 1 (20%) asthma, 3 none | 5 fever, 5 cough, 4 malaise/headache, 1 dyspnea | 4 pts <50; 1pt. 45,500 | 563.7 | Y/80% | 5 | 0 |
| Childs et al. (no=18) | 6 (33.3%) HTN, 4 (22.2%)DM, 5 (27.8%) CKD, 10 (55.6%) obesity | 11 Fever, 13 cough, 12 SOB | <50 | 395 | Y/100% | 18 | 5 |
| Ridgeway et al. (no= 5) | 2 (40%)HTN, 1 (20%) DM, 1 (20%) HLD, 3 (60%) obesity, 1 (20%) OSA, 1 (20%) TB, 1 (20%) Addison’s dis, 1 CHF, 1 COPD, 1 PE | 3 fever, 4 cough, 3 SOB; 3 diarrhea, 2 myalgia, 2 headache, 2 n/V, 1 abd pain, 1 chest pain, 1 dyspnea | 20 | 331 | Y/100% | 5 | 0 |
| Byrd et al. (no=27) | N/A | cough, dyspnea, lethargy, fever, headache, sore throats, chest pain, myalgia, anosmia | <200 | 87-1441 | Y/n/a | 9 | 1 |
| Altuntas aydin et al. (no= 4) | 1 (25%) DM, 1 (25%) COPD, 1 (25%) HTN, 1 (25%) HBV, 1 (25%) bipolar disorder, 1 (25%) obesity (35.5 BMI) | Dyspnea, cough, fever, diarrhea | N/A | 627 | Y/100% | 4 | 0 |
| Marimuthu et al. (no= 6) | 2 (33.3%) HTN | 5 fever, 2 cough, 1 sore throat | N/A | 535 | Y/83.3% | 6 | 0 |
| Calza et al. (no= 26) | 11 (42.3%) HTN, 4 (15.4%) DM, 4 (15.4%) obesity, 3 (11.5%) asthma / chronic respiratory disease, 3 (11.5%) hypothyroidisms | 19 Fever, 14 cough, 11 fatigue, 11 myalgia, 3 dyspnea, 4 tachypnea | <50 | 350 | Y/n/a | 5 | 0 |
| Zhu et al. (no= 1) | DM2, smoker | Fever, cough, SOB | N/A | N/A | N | 1 | 0 |
| Zhao et al. (no=1) | None | Fever, myalgia | <500 | 216 | Y/100% | 1 | 0 |
| Wang et al. (no=1) | None | Fever, cough, chest pain | N/A | 34 | N/A | 1 | 0 |
| Kiran Rashed et al. (no=1) | CAD, DM | Fever, cough, dyspnea, myalgia | Und. | 266 | Y/100% | 1 | 0 |
| Toombs et al. (no=3) | 2 (66.7%) DM, 2 (66.7%) HTN, 2 (66.7%) smoker, 1 (33.3%) TB, 1 (33.3%) obesity, 1 (33.3%) Graves’ disease | 2 Fever, 3 cough, 2 dyspnea, 1 headache, 1 anorexia | -2 pt.: und. | 373 | Y/100% | 3 | 1 |
| -1 pt.: 1,000,000 | |||||||
| Baluku et al. (no=1) | N/A | Headache, chest pain, myalgia, anorexia | <1000 | 965 | Y/100% | 1 | 0 |
| Nakamoto et al. (no=1) | HBV, syphilis | Cough | 100 | 194 | N/A | 1 | 0 |
| Hadded et al. (no=1) | None | Fever, cough, altered mental status, GI (vomiting. Abd. pain) | Und. | 604 | Y/100% | 1 | 0 |
| Menghua et al. (no=1) | h/o syphilis (cured) | Fatigue | Und. | 224 | Y/100% | 1 | 0 |
Table 5: Patient Characteristics of all reviewed articles (comorbidity, CD4 counts, viral load).
Discussion
We conducted a systematic quantitative review to evaluate certain aspects of COVID-19 in patients living with human immunodeficiency virus (PLWH). The outcomes were categorized into risk of hospitalization, ICU admission, IMV requirement and mortality. The review of 23 articles indicated that there was a consistent high risk of hospitalization, ICU admission, IMV requirement and mortality in PLWH+ confirmed COVID-19- positive in the cohort studies, case series and case reports. The rate of hospitalization from the pooled data of the 3 groups (Ch, CS, Cr) was 69.13% (n=450); 70.49% in Ch [12-18], 57.14% in CS [19-25], and 100% in CR [26-34]. The rate of ICU admission was 12.9% (n=84) for the 3 groups; 18.61% in Ch [12-18], 17.31% in CS [19-25], and 27.27% in CR [26-34]. And the overall case fatality rate of all reviewed articles was 11.21% (n=73).
Our results indicate that PLWH were a high risk group with greater than 50% needed hospitalization while 1/5 of hospitalized patients needed critical care. This finding aligns with the argument that predicts a bad outcome of COVID-19 in PLWH due to immunocompromised status [14,20] and does not support the argument predicting a better prognosis for PLWH [15,17-19]. The better prognosis could be explained by a lower risk for cytokine storm as a result of immunodeficiency, as there are low CD4 T lymphocytes which are responsible for activating innate immune cells like B-lymphocytes, cytotoxic T cells, and non-immune cells. There is also some suppression of the immune reaction through subtype T helper cells [35,36].
Correlation analysis of CD4+ counts with hospitalization and mortality were not consistent in all reviewed studies as it showed weak positive in case series and case reports while weak negative in cohorts [12,14-18]. And in mortality, there was a weak negative correlation in cohorts and case series [19-25] while weak positive in case reports [26-34]. More studies needed to focus on this to determine if CD4+ counts strongly correlate with the outcome of COVID-19.
We found that PLWH and confirmed COVID-19 do not present differently than the rest of the population as the common clinical presentations are cough (28.2%), fever (27.3%), and shortness of breath (10.8%), which is consistent with the finding of cohorts [12,14,16-18] and case series [19-25].
Analyzing the available data for deceased patients, we found the age range to be between 47 and 82 with a male to female ratio of 3:1, this correlates with the general population as data reveal that males had a mortality rate more than females [37]. The comorbidity data of deaths were available for 26 deceased individuals: HTN (no=9), DM (no=6), COPD (no=3), CKD (no=8), ESRD (no=1), CVD/ CHF (no=2), organ transplant (no=1), prior stroke (no=2), cancer (no=2), liver cirrhosis (no=1), and obesity (no=3). The CD4+ counts ranged from below 50 to 800. The average CD4+ counts for Ho et al. [14], Meyerowitz et al. [16], and Harter et al. [18] of the deaths the cohort studies were 686, 426, and 389, respectively, and mortality rates in these cohorts were 20.5%, 5.6%, and 9.1%, respectively.
Nearly 98% of the cohort and 95% of the case series patients were being treated with ART at the time of diagnosis. According to Amo et al. [13], HIV-positive patients who were receiving TDF/FTC had a lower risk of contracting COVID-19 and ultimately being hospitalized, concluding that further investigation is necessary. However, Kate et al. [20] concluded that patients on suppressive ART were not protected from moderate or severe COVID-19. We could not find any correlation between a specific type of ART and the risk of hospitalization, ICU admission, or mortality.
Despite the fact that this review was done following a standardized methodology, it presents with specific limitations. Cohort studies that we reviewed, such as Amo et al. and Meyerowitz et al. did not report an HIV viral load which makes it difficult to assess in relation to the other cohorts. Amo et al. also did not disclose a CD4+ count. Among the case reports, the HIV viral load and the total number of patients on IMV were not reported by Maomao et al. Lastly, Byrd et al. did not present the total number of patients admitted in the ICU and the number of patients on IMV. We did not run a study assessment because these limitations create barriers which make it hard to assess and compare the studies with one another.
Conclusion
In conclusion, our results indicate that PLWH with COVID-19 are at increased risk for hospitalization, ICU admission, IMV requirement and mortality. We recommend classifying this group as high risk and they should adhere strictly to COVID-19 precautions. Authors conclude that larger sample size and study volume is needed to fully evaluate the outcome of this novel virus and confirm the association of CD4+ cell counts with severe forms of the disease and mortality.
REFERENCES
- Listings of WHO’s response to COVID-19. Who.int. 2021.
- Zhu N, Zhang D, Wang W. A Novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733.
- Zhou P, Yang X, Wang X, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273.
- Kankyokansen. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. 2020.
- Carrillo-Larco RM, Altez-Fernandez C. Anosmia and dysgeusia in COVID-19: A systematic review. Wellcome Open Res. 2020;5.
- Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet. 2020;8(5):475-481.
- Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney impairment is associated with in-hospital death of COVID-19 patients. MedRxiv. 2020.
- Lauer SA, Grantz KH. Qifang Bi, Forrest K Jones, Qulu Zheng, Hannah R Meredith, et al. The incubation period of coronavirus disease 2019 (covid-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577-582.
- Zhang XW, Yap YL. Structural similarity between HIV-1 gp41 and SARS-CoV S2 proteins suggests an analogous membrane fusion mechanism. Theochem. 2004;677(1-3):73-76.
- Ritonavir and Other HIV Protease Inhibitors. Coronavirus Disease COVID-19. Nih.gov.
- Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PloS One. 2020;15(8):e0238215.
- Vizcarra P, Pérez-Elías MJ, Quereda C, Moreno A, Vivancos MJ, Dronda F et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7(8):e554-564.
- Del Amo J, Polo R, Moreno S. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med. 2020.
- Shalev N, Scherer M, LaSota ED, Antoniou P, Yin MT, Zucker J, et al. Clinical characteristics and outcomes in people living with HIV hospitalized for COVID-19. J Infect Dis. 2020.
- Sigel K, Swartz T, Golden E, Paranjpe I, Somani S, Richter F, et al. Coronavirus 2019 and people living with human immunodeficiency virus: outcomes for hospitalized patients in New York City. Clinical infectious diseases. 2020;71(11):2933-2938.
- Meyerowitz EA, Kim AY, Ard KL, Basgoz N, Chu JT, Hurtado RM, et al. Disproportionate burden of coronavirus disease 2019 among racial minorities and those in congregate settings among a large cohort of people with HIV. AIDS. 2020;34(12):1781.
- Gervasoni C, Meraviglia P, Riva A, Giacomelli A, Oreni L, Minisci D, et al. Clinical features and outcomes of patients with human immunodeficiency virus with COVID-19. Clin Infect Dis. 2020;71(16):2276-2278.
- Härter G, Spinner CD, Roider J, Bickel M, Krznaric I, Grunwald S, et al. COVID-19 in people living with human immunodeficiency virus: A case series of 33 patients. Infection. 2020:1.
- Blanco JL, Ambrosioni J, Garcia F, Martínez E, Soriano A, Mallolas J, et al. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020;7(5):e314-e316.
- Childs K, Post FA, Norcross C, Ottaway Z, Hamlyn E, Quinn K, et al. Hospitalized patients with COVID-19 and human immunodeficiency virus: A case series. Clin Infect Dis. 2020;71(8):2021-2022.
- Ridgway JP, Farley B, Benoit JL, Frohne C, Hazra A, Pettit N, et al. A case series of five people living with HIV hospitalized with COVID-19 in Chicago, Illinois. AIDS Patient Care STDS. 2020;34(8):331-335.
- Byrd KM, Beckwith CG, Garland JM, Johnson JE, Aung S, Cu‐Uvin S, et al. SARS‐CoV‐2 and HIV coinfection: Clinical experience from Rhode Island, United States. . J Int AIDS Soc. 2020;23(7):e25573.
- Altuntas Aydin O, Kumbasar Karaosmanoglu H, Kart Yasar K. HIV/SARS‐CoV‐2 coinfected patients in Istanbul, Turkey. J Med Virol. 2020;92(11):2288-2290.
- Janakiram Marimuthu DB, Gandhi PA. HIV and SARS CoV‐2 co‐infection: A retrospective, record based, case series from South India. J Med Virol.2020.
- Verucchi GR. Leonardo Calza, Isabella Bon, Marina Tadolini, Marco Borderi, Vincenzo Colangeli. COVID-19 in patients with HIV-1 infection: a single-centre experience. Infection. 2020;1-5.
- Zhu F, Cao Y, Xu S, Zhou M. Co‐infection of SARS‐CoV‐2 and HIV in a patient in Wuhan city, China. J Med Virol. 2020.
- Zhao J, Liao X, Wang H, Wei L, Xing M, Liu L, et al. Early virus clearance and delayed antibody response in a case of coronavirus disease 2019 (COVID-19) with a history of coinfection with human immunodeficiency virus type 1 and hepatitis C virus. Clin Infect Dis. 2020;71(16):2233-235.
- Wang M, Luo L, Bu H, Xia H. One case of coronavirus disease 2019 (COVID-19) in a patient co-infected by HIV with a low CD4+ T-cell count. Int J Infect Dis. 2020;96:148-150.
- Mahmood K, Rashed ER, Oliveros E, Chau VQ, Hermle T, Jacobs S, et al. Predisposition or protection? COVID-19 in a patient on LVAD support with HIV/AIDS. Case Rep. 2020;2(9):1337-1341.
- Toombs JM, Van den Abbeele K, Democratis J, Merricks R, Mandal AKJ, Missouris CG. COVID-19 in three people living with HIV in the United Kingdom. J Med Virol. 2020;10(2):1002.
- Baluku JB, Mwebaza S, Ingabire G, Nsereko C, Muwanga M. HIV and SARS‐CoV‐2 co‐infection: A case report from Uganda. J Med Virol. 2020.
- Nakamoto T, Kutsuna S, Yanagawa Y, Kanda K, Okuhama A, Akiyama Y. A case of SARS-CoV-2 infection in an untreated HIV patient in Tokyo, Japan. J Med Virol. 2020.
- Haddad S, Tayyar R, Risch L, Churchill G, Fares E, Choe M, et al. Encephalopathy and seizure activity in a COVID-19 well controlled HIV patient. IDCases. 2020;21:e00814.
- Menghua W, Xin Z, Jianwei L, Yu Z, Qinwei Y. Case report: One case of coronavirus disease 2019 (COVID-19) in a patient co-infected by HIV with a normal CD4+ T cell count. AIDS Res Ther. 2020;17(1):1-4.
- Mascolo S, Romanelli A, Carleo MA, Esposito V. Could HIV infection alter the clinical course of SARS-CoV-2 infection? When less is better. J Med Virol. 2020; 92: 1777-1778.
- Rishi VL, Rui Z, Asha DV, Bing X. CD4⁺ T Cells: Differentiation and Functions. Clinical & developmental immunology. 2012;925135.
- Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81(2):e16-e25.
Citation: Sheikh M, Nagpal S, Zaidi M, Vijayan R, Matos W, Ngaba NN, et al. (2021) Influence of Novel Coronavirus COVID-19 and HIV: A Scoping Review of Hospital Course and Symptomatology. J Vaccines Vaccin. 12:461.
Copyright: © Sheikh M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

