Indexed In
- Open J Gate
- Genamics JournalSeek
- ResearchBible
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 12, Issue 2
Improving Doxorubicin-Chemotherapy Treatment with Luteolin and Resveratrol: A Novel Synthetically Engineered Secondary Metabolite "TDB-13" Created with Luteolin and Resveratrol
Jessie Dong*Received: 08-Dec-2022, Manuscript No. BOM-22-19143; Editor assigned: 12-Dec-2022, Pre QC No. BOM-22-19143 (PQ); Reviewed: 26-Dec-2022, QC No. BOM-22-19143; Revised: 02-Jan-2023, Manuscript No. BOM-22-19143 (R); Published: 09-Jan-2023, DOI: 10.35248/2167-7956.23.12.261
Abstract
First-line chemotherapy drug doxorubicin, the most potent chemotherapy drug to date, is used in virtually all chemotherapy treatment plans. 92% of cancer patients are treated with chemotherapy yet for the past six decades, chemotherapy has had a failure rate of 90%. An overwhelming majority of the failures are attributed to the side effects of doxorubicin. No existing treatment exists that mitigates doxorubicin’s repercussions without significantly depleting its therapeutic efficacy.
While research indicates that secondary metabolites are improved when working with other chemicals/compounds and that luteolin and resveratrol specifically have protective effects on heart tissue (which could alleviate a major side effect: cardiotoxicity), no research has tested any secondary metabolites on any chemotherapy drug. To evaluate therapeutic efficacy: luteolin, resveratrol, and doxorubicin treated in vitro models of carcinoma (80%-90% of all cancer cases) alone and as a trio. To test the side effect of cardiotoxicity: Extracellular matrix components were coated onto the surface of cardiomyocytes.
Results of luteolin and resveratrol alone indicate that though they are therapeutic to in vitro carcinoma cells, there is one weakness: A small therapeutic window (concentrations of 15 μM and 20 μM being equally or less effective as the lowest concentrations of 5 μM and 10 μM) — suggesting that while luteolin and resveratrol have increased in popularity (in the form of dietary supplements) among cancer patients by 82% since 2010, the compounds may not always produce the desired effect. Combining luteolin and resveratrol with doxorubicin was able to improve therapeutic efficacy of doxorubicin while reducing cardiotoxicity. However, the weakness of a small therapeutic window still existed. By methylation and glycosylation of both luteolin and resveratrol, a novel compound that the present study named “TDB-13” was able to maintain the level of therapeutic efficacy and reduction of cardiotoxicity while lengthening the therapeutic window. Thus, new components to chemotherapy treatment can potentially improve it greatly.
Keywords
Luteolin; Chemotherapy; Cardiotoxicity; Doxorubicin; Treatment
Introduction
American cancer patients and long-term cancer survivors use dietary supplements, functional foods, and nutraceutical significantly more than Americans without cancer (64% to 81% more) [1]. Cancer patients are also more vulnerable to the potential side effects of these unregulated products such as rashes, shortness of breath, severe muscle pain, and slurred speech [2] than healthy individuals.
With increasing numbers of cancer patients using products derived from secondary metabolites luteolin and resveratrol, more research is needed to determine whether the self-prescribed products are actually advantageous. While some research indicates that flavonoids like luteolin and stilbenoids like resveratrol have anti- inflammatory properties that alleviate symptoms of carcinomas, other research indicates that excessive intake of certain groups of natural compounds such as flavonoids can produce toxic effects [3]. Some researchers have coined the term “flavonoid toxicity” after reports of toxic flavonoid-drug interactions that caused liver failure, contact dermatitis, hemolytic anemia, and estrogenic-related concerns [4]. It is likely that they act as mutagens, inhibitors of key regulatory enzymes, or as pro-oxidant molecules [5].
The side effects of chemotherapy provide an opportunity to repurpose luteolin and resveratrol in direct cancer treatment rather than indirect cancer treatment (through cancer patients self- prescribing products). Currently, 92% of cancer patients go through chemotherapy [6]. Yet, for the past six decades, chemotherapy has consistently had a failure rate of greater than 90%; many of these failures (death) are attributed to the side effects of chemotherapy [7].
Chemotherapy drugs are injected into patients through their bloodstream through central lines, PICC lines, and portacaths [8]. The most widely used chemotherapy drug since the 1960s for all cancers is doxorubicin (also known as adriamycin) the most potent chemotherapy to date. It is an anthracycline developed through the isolation from cultures of Streptomyces peucetius variety caesius [9]. It slows or stops the growth of cancer cells by blocking the enzyme known as topoisomerase 2 [9]. Doxorubicin is a first-line chemotherapy drug; there are virtually no chemotherapy treatment plans that do not involve doxorubicin [10].
As chemotherapy is a method to treat various cancers, researchers have established the usage of general cancer types rather than a specific cancer if the experiment is centrally about the delivery of chemotherapy. One major cancer type is carcinoma, a type of cancer that forms in epithelial tissue-a tissue that lines most of your organs, the internal passageways in your body (like your esophagus), and your skin [11]. Past research has indicated that when in vitro models are created for chemotherapy, one general cancer type that is most representative of real chemotherapy reactions in the human body is carcinoma. 80% to 90% of all cancer cases are carcinomas; most cancers affecting your skin, breasts, kidney, liver, lungs, pancreas, prostate gland, head and neck, are carcinomas [12].
Regardless of whether doxorubicin is used alone or with other drugs, the side effects are severe and common. Some examples include increased risk of getting an infection, breathlessness, bruising, bleeding gums, extreme fatigue, hair loss, loss of appetite, infertility, growth of heart and liver abnormalities, growth of new tumors, and even permanent DNA damage [13]. Doxorubicin’s potency poses a problem because currently, it is recommended that the cumulative total lifetime dose of doxorubicin be under 450 mg/ m2 to 550 mg/m2 [14]. Above this dosage, the risk of irreversible congestive cardiac failure, myelosuppression and palmar pla`ntar erythrodysesthesia drastically increases [14]. However, carcinoma patients often go through more than seven rounds of chemotherapy treatment, each of which include a dosage of 60 mg/m2-95 mg/m2 [15] thus, a majority of cancer patients are in danger of exceeding their recommended lifetime dose of doxorubicin.
In the past, researchers have experimented by changing doxorubicin delivery methods. One example is packaging doxorubicin inside fat particles in hopes that the human body will absorb it with fewer side effects [16]. Researchers have tried to incorporate doxorubicin into nanoparticles in order to reduce total drug use [17]. Unfortunately, past tested delivery methods have not been successful. Additionally, there has been extremely limited research on improving doxorubicin because pharmaceutical companies fund most drug research and there is no profit in spending money to change doxorubicin when the drug is still being widely purchased [18]. There is only one drug in clinical use that can protect against doxorubicin-induced cardiotoxicity and doxorubicin-induced tumor formation-dexrazoxane. Unfortunately, dexrazoxane significantly depletes the anti-tumor effect of doxorubicin so much that it virtually removes all positive effects of doxorubicin [19]. Further, dexrazoxane increases the incidence of secondary malignancies [19]. Therefore, a major problem in cancer research that is yet to be solved is to reduce doxorubicin’s toxicity without significantly depleting its therapeutic efficacy.
While research has looked at combining natural secondary metabolites for a synergistic effect on preventing cancer [20] no research has investigated the interaction of secondary metabolites and a chemotherapy drug on treating cancer. Given that research with doxorubicin has indicated that combination treatment with other chemotherapy drugs reduces side effects of cardiotoxicity and inflammation, it is possible that doxorubicin will work better when combined with other agents that have the ability to alleviate its negative effects. In the past, the small-molecule inhibitor Gamitrinib suppressed some unwanted side effects, notably the emergence of new tumors [21]. Trastuzumab (also known as Herceptin, Herzuma, and Ontuzant), a targeted chemotherapy drug, and doxorubicin have been used together the past two decades [22] and through biomarker evaluations, researchers have found that doxorubicin and trastuzumab administered alone because more heart problems and heart-related side effects than when administered together [23].
One piece of evidence that supports the idea that the secondary metabolites luteolin and/or resveratrol may have this ability is that they are strong antioxidants. Studies have shown that doxorubicin causes myocardial damage by blocking the mechanism of antioxidant cells which in turn causes the accumulation of reactive oxygen species and increases the apoptosis of myocardial cells [24]. Additionally, luteolin and resveratrol have been shown to exert a protective effect in heart injury models by protecting heart tissues in diabetic mice by modulating Nrf2-mediated oxidative stress and NF-κB-mediated inflammatory responses [25].
It would be greatly beneficial if these two natural secondary metabolites have a therapeutic effect on cancer. Cancer remains the leading cause of death worldwide and based on 2015 through 2017 data; approximately 39.5% of all people will be diagnosed with cancer at some point during their lives. Carcinoma malignancies account for 80% to 90% of all cancer cases and are very serious [26]. Carcinomas of the skin often easily destroy healthy tissue, spread to the lymph nodes and other vital organs (World Health Organization, 2022). In fact, carcinoma malignancies are the most common type of cancer that metastasizes throughout the body (World Health Organization, 2022).
The first objective of the present study is to test luteolin and resveratrol’s therapeutic efficacy. The second objective is to create a method to improve doxorubicin in a way that preserves its strong therapeutic efficacy while suppressing unwanted side effects. These will be tested through two parts in vitro cellular models that measure cytotoxicity and cardiotoxicity, and the synthetic production of a new compound through combination, methylation, and glycosylation of luteolin and resveratrol.
Methodology
Experiment 1: The effect of luteolin on carcinoma cells; the effect of resveratrol on carcinoma cells
To explore how luteolin and resveratrol behave, in vitro models were used. In vitro models are widely regarded as a reputable approach to model cancer [27]. There are several moving parts in cancer development and progression which makes in vitro models valuable because they can simulate each feature of the complex path of cancer development and progression. For instance, the invasion and metastasis of cancer occurs when tumor cells disseminate from the primary tumor through body systems (such as the circulatory and lymphatic systems), then invade across membranes and endothelial walls, and finally colonize distant organs [28]. Cell migration and adhesion are vital steps in this process and therefore, the present research takes these steps into account through in vitro experiments.
Organization of cell plate: Cell culture plates were purchased from Costar in Cambridge, Massachusetts.
Pre-treatment and preparation of secondary metabolites: In Experiment 1, there were seven conditions (including the control of just the carcinoma cells without treatment) for each secondary metabolite. 1 mg of luteolin and 1 mg of resveratrol, labeled as 98% pure, were received from Apothex Products. To create solutions, each compound was diluted in Minimum Essential Medium (MEM) to concentrations (initially) of 0.1 μM, 1 μM, 5 μM, 10 μM, 15 μM, and 20 μM. For each compound, 900 μL of MEM was added to three separate 1.5 ml tubes via micropipette. 1000 μL of Dimethyl Sulfoxide (DMSO) was added into the bottles with 1 mg of luteolin and 1 mg of resveratrol. 100 μL of this solution was placed into the first tube and the mixture was vortexed to dissolve the powder into the solvent. The solution was diluted tenfold by adding 100 μL of the 100 μM solution of the compound to the second tube and hundred-fold by adding 100 μL of the 10 μM solution to the third tube. All solutions were refrigerated overnight at 4°C.
General carcinoma cancer cell preparation: As the goal is to explore chemotherapy rather than a specific type of cancer, the general carcinoma cell line widely used to do research on chemotherapy- HCC70-was used. Carcinomas are the most common group of cancer found in the skin, breasts, kidney, liver, lungs, pancreas, prostate gland, head and neck. Patient-derived HCC70 was initially stored inside three flasks, each containing media. After removing all waste media, the cells inside the flask were trypsinized with 4 ml of trypsin solution (0.25% trypsin EDTA) per flask and left to wait for 4 minutes. To neutralize the trypsin, 4 ml of media was added to each flask. Each solution was transferred into one 1.5 ml tube and centrifuged at 3,000 RPM for 5 minutes. All supernatants were removed. With only the cell pellet, 6 ml of media was added to each tube and mixed with a pipette. From these tubes, the cells were plated into 6-well plates, 24-well plates, and 96 well-plates. All plates were incubated at 37°C at a CO2 level of 5%.
Cytotoxicity assay: The type of cytotoxicity assay used was the MTT assay. MTT assays are regarded as a reliable way to determine cytotoxicity and cell viability through metabolically active cells reducing yellow-colored MTT to purple formazan crystals [29]. For each cancer, an MTT assay was performed. Following preparation and incubation of a 96-well flat bottom plate, 5 mg of yellow tetrazolium MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) powder was diluted with 1.0 ml Phosphate Buffered Saline to ensure an isotonic environment. After vortexing until the MTT dissolved, 10 μL of the MTT labeling reagent was added in order to assess the activity of NADPH-dependent cellular enzymes within cells. The plate was incubated for 1.5 hours at 37°C at a CO2 level of 5%. 40 μL of media was drained from each well and due to some cells respirating; formazan formed which needed to be dissolved. Hence, 80 μL of DMSO was added to each well for the solubilisation formazan. After the plate rested for 10 minutes, the plate was inserted into a sandwich Enzyme-Linked Immunosorbent Assay (ELISA) Biorad iMark Microplate reader with a wavelength of 595 nanometers. The reader spectrophotometrically evaluated the resulting solution to determine Optical Density (OD) of the developed color.
Cell viability rates were calculated for each MTT assay using the formula 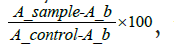 where A_b is the blank value and equals 0.05.
where A_b is the blank value and equals 0.05.
Metastasis
Colony formation: A 6-well plate was seeded with carcinoma cell cultures, and treated using 10 μL of luteolin and 10 μL of resveratrol. One well was designated as the control group and given no treatment. Subsequently, the cells were given 18 days for treatment, with a change of medium and retreatment using the treatments after the first and second week. The same staining procedure for the cell migration assay was used: 1.0 mL of a methanol was added to each well to preserve the cells for microscopic examination, and after approximately 2 minutes, removed. The same procedure was repeated for Crystal Violet dye. Finally, to fully remove the stain, each well was washed out using water twice, each time for only about 10 seconds. Once the 2 minutes allotted to drying the plate passed, it was placed under a microscope at 40X magnification and ImageJ was used to take 10 pictures of each of the different concentrations.
Cellular adhesion: Cell adhesion is the binding of a cell to the Extracellular Matrix (ECM), other cells, or another surface, is vital for the growth and survival of the cell and its communication with other cells. In a human body, a strong ability to adhere to surfaces oftentimes means a stronger metastatic ability of cancer cells. To amplify, increased cell attachment is a pro-cancer hallmark and indicates that the cells have increased ability to attach to one another, grow, and then migrate to a different location. Therefore, a level of attachment as a measurement was used as a hallmark for metastasis.
The adhesion assay used measured the level at which cells express integrin, a transmembrane receptor that facilitates cell-extracellular adhesion. For each patient-derived cancer cell line, a 24-well tissue culture plate was prepared. Each well was coated with 2 μL of fibronectin substrate to create a protein layer. To ensure that each well had an equal amount of cells, a hemocytometer was used to bring the cell media up to the optimal density of (5 × 106)/mL. 0.5 mL of suspended cells were added to each well and treated with 5 μL of decreasing concentrations of the compound of interest. For the positive control, three wells were prepared with only the compound and for the negative control; three wells were set with no treatment. The 24-well plate was placed in an incubator at 37°C at 5% carbon dioxide for 24 hours.
For each adhesion assay, unattached cells and their solution medium were removed from the wells through the technique of aspiration. Thereby, the attached cells are still on the plate and were stained with 500 μL of Hema-3 fixative to stain the cells with a blue dye. This took approximately one minute. The staining fluid was removed with a micropipette after one minute. I then added another 500 μL of Hema-3 fixative stain solution to each well to color the remaining cells that may not have been stained the first time to ensure that all cells in the well are being accounted for. 500 μL of distilled water is added to each well to remove excess staining after the fixative stain solution stays in each well for approximately one minute. Each well was rinsed using a micropipette and water from each well was expelled and then the plate was left to dry before analysis.
After the plate had dried, pictures of the cells were taken using the 4X magnification attachment of an Am Scope scanning microscope. The images were processed and cells were counted within each well using ImageJ software. The image was converted into an “8-bit” image. To remove image coloring and excess background shadows, the substitution technique on the software was used to create an optimal image for cell counting. The images were processed with the threshold function to distinguish the cells from the remaining background. The images were despeckled to remove remaining particles that are not cells and also regions that the software regards as “noise”. The Image-based Tool for Counting Nuclei (ITCN) plugin automatically counts the number of cells within an image through three inputs: an estimation of the diameter of a cell, an estimation of the minimum distance between cells, and either a Region Of Interest (ROI) selected with ImageJ's selection tools or a black and white mask image that is white in regions that are to be counted. The ITCN plugin to count the cells was run under the framework of a 10 μL width function. The number computed was averaged with other photos of the same well in order to determine the final cell count.
Cellular migration: The cell cultures were added to a 6-well plate. 10 μL of the natural compound of interest were added to 5 of the wells in various concentrations and the remaining well was designated as the control group. Then, each well had 3 horizontal lines scratched onto the bottom using a 200-μL Pipette tip to stimulate the wounds. After an incubation period of 72 hours at 37°C with 5% carbon dioxide levels, all the media was withdrawn. 1.0 mL of Methanol was added to each well to preserve the cells for microscopic examination, and after approximately 2 minutes, removed. The same procedure was repeated for Crystal Violet dye. Finally, to fully remove the stain, each well was washed out using water twice, each time for only about 10 seconds. Once the 2 minutes allotted to drying the plate passed, it was placed under a microscope at 40X magnification, and the 3 lines were observed. ImageJ was used to take 6 pictures of each of the different concentrations, with a considerable focus on the cells located in between the lines. The number of cells was then counted using the Image-based Tool for Counting Nuclei (ICTN) plugin.
Following the incubation, the staining and cleaning procedure proceeded as usual. ImageJ was used to take 6 pictures of each of the different concentrations, with a considerable focus on the wounds themselves. Using the pictures, the length of each rift was measured to see how much the cells had grown back. The span of the rifts was inversely proportional to the survival and continuation of growth in the cells.
Data analysis
For many of the cellular assays, there were three trials and rather than averaging all of the values, at least 18=data analysis values were inputted into JASP statistics software which then computed the standard deviation. Then, the values JASP output were manually inputted to GraphPad Prism 6.0. The standard deviation from the mean is visually depicted on the graphs. The present study uses the following symbols: ns=not significant, *=p<0.05 compared to control, **=p<0.01 compared to control, ***=p<0.001 and #=p<0.05 compared to each other.
Experiment 2a: Luteolin and resveratrol reducing cardiotoxicity (an unwanted side effect of doxorubicin) while preserving doxorubicin’s cytotoxicity in carcinoma cells
Doxorubicin has been the dominating first-line chemotherapy drug for all cancers since the 1960s [30] thus, it is well-documented that it prevents metastasis both in vitro and in vivo [31]. The researcher did not feel the need to repeat these experiments which is why the three “modeling metastasis” models used in the method of “objective 1” were not used in “objective 2”. Instead, this part of the method focuses on reducing cardiotoxicity in heart cells yet ideally still preserving its cytotoxicity in carcinoma cells.
Pre-treatment and preparation of doxorubicin: Doxorubicin in a purity of ≥ 98% was purchased from Cayman Chemicals, with the only ingredient listed being the formal name of the drug (8S,10S)- 10-[(3-amino-2,3,6-trideoxyα-L-lyxo-hexopyranose)oxy]-7,8,9,10- tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-5,12- naphthalenedione, monohydrochloride). The same serial dilution procedure aforementioned in the method of “Experiment 1” was followed, resulting in concentrations of 0.1 μM, 1 μM, 5 μM, 10 μM, 15 μM, and 20 μM of luteolin, resveratrol, and doxorubicin. When testing combination treatments, the individual molar concentrations would be split in half in order to end up with equal parts of each component.
Healthy human-derived cell preparation: Human-derived ACS- 1030 was initially stored inside three flasks, each containing media. After removing all waste media, the cells inside the flask were trypsinized with 4 mL of trypsin solution (0.25% trypsin EDTA) per flask and left to wait for 4 minutes. To neutralize the trypsin, 4 mL of media was added to each flask. Each solution was transferred into one 1.5 mL tube and centrifuged at 3,000 RPM for 5 minutes. All supernatants were removed. With only the cell pellet, 6 mL of media was added to each tube and mixed with a pipette. From these tubes, the cells were plated into 6-well plates, 24-well plates, and 96 well-plates. All plates were incubated at 37°C at a CO2 level of 5%.
Measuring cardiotoxicity through cardiomyocytes (HiPSCs): Cardiomyocytes were used and extracellular matrix components were coated onto the heart cells.
Finding luteolin and resveratrol’s potential mechanisms of action:
In silico virtual screening: In silico virtual screening via molecular docking is used to simulate the binding between a ligand and a receptor which then generates a binding affinity for each ligand- receptor interaction. Molecular docking can be useful to gain insight about the various binding modes of a ligand (luteolin) with a protein. Knowing the binding affinities allows for a foundation to test which binding modes are optimized to observe the relationship between a ligand and a protein.
Preparation of ligands: Lists of potential chemical ligands for screening were compiled after conducting a literature search on PubMed and using the software BioChem to find molecular descriptors and fingerprints. To do this, OEChem’s 2021 Simplified Molecular Input Line Entry System (SMILES) in the format C1=CC(=C(C=C1C2=CC(=O)C3=C(C=C(C=C3O2)O)O)O)O was utilized. Then, a finalized list of seven ligands to be processed were created and downloaded from the PubChem database in a Spatial Data File (SDF) 2-dimensional format. The PubChem CID of luteolin is 5280455 and 445154 for resveratrol. The online Simplified Molecular Input Line Entry System (SMILES) translator and structure file generator was used and in this generator, the “Kekule Structure” was chosen in order to easily tell the nature of electron bonds. In the “Kekule Structure”, bonded electron pairs are in covalent bonds represented as lines and non-bonded electron pairs are dots in a Lewis Structure. The Spatial Data File (SDF) was converted into a Program Database (PDB) and the 3-dimensional option was chosen in order to finalize the translation.
Preparation of the macromolecules: 28 macromolecules were taken from the RCSB Protein Data Bank. Since some of the macromolecules downloaded had other macromolecule(s) in complex with it, BioVia was utilized to remove the macromolecules that were in complex with the proteins that did not come in their crystal structure.
Utilizing PyRx autodock vina: Molecular docking was performed through PyRx, a version of the AutoDock Vina program by the National Biomedical Computation Resource. In PyRx, the OpenBabel option was selected. The ligand, either luteolin or resveratrol, was inserted in a mimized PDB format. The ligand was converted into “Autodock Vina” and the macromolecules were uploaded in the “molecules” section. In Vina Wizard, the maximize option was selected and the binding affinity process was processed under grid box dimensions of the x, y, and z conformations fixed at 25, 25, and 25. After docking each pair with a ligand and a macromolecule, the interactions between them were shown visually through Discovery Studio Visualizer version 21.01.0.20298. The equation used was:

Note: EP-binding energy; epair-electron pair; d-surface distance, Ri- radius of atom 1 in electron pair; Rj-radius of atom 2 in electron pair; Gauss1-first attractive gaussian term to mimic a Lennard- Jones energy profile and set a first minimum for steric interactions; Gauss2-second attractive gaussian term to mimic a Lennard-Jones energy profile and set a first minimum for steric interactions; Repulsion-force of separation between two atoms; Hydrophobic- force of attraction between nonpolar molecular surfaces; Hbond- force of attraction between a hydrogen and an electronegative atom.
Quantifying results of in silico screening: Luteolin and resveratrol were tested separately. For each, one 6-well plate was used and labeled by sample name and concentration. 50 μL of caspase assay buffer was added into each well. Afterwards, 45 μL of caspase lysis buffer was added to each well. Then, 10 μL of each of the samples that were prepared previously were matched to corresponding labels and then added into wells using an adjustable volume pipette. Then, 5 microliters of caspase substrate was added to each well. For the caspase assay, a 6-well plate was prepared containing for each of the desired cell lines and 10 μL of each treatment was added into each well. Afterwards, the plate was placed back into the incubator for one day to allow the cells to react to the chemical. The following day, the contents of each well was put into plastic tubes labeled with each corresponding treatment. Each of these tubes was then inserted into the centrifuge and spun for three minutes to clump all the cells near the bottom of the tubes. Next, all the media had to be removed from each tube via a pipette leaving only the cells at the bottom. Following this, 50 μL of lysomic buffer was added and the cells were mixed evenly throughout the fluid. Afterwards, a 96 well plate was prepared by labeling the wells with each corresponding treatment. Then, 50 μL of each treatment was added into each well alongside 45 μL of cell lysis buffer. Finally, a 5 μL caspase assay buffer was introduced to detect the presence of caspase. In order to get results, a spectrophotometer at 405 nanometers was used to measure the reactivity of the caspase to the caspase assay buffer. Thus, if the caspase assay buffer was highly reactive, it indicates the approximate amount of cellular apoptosis. The cells were then run through the spectrophotometer for 15-minute intervals until one hour had passed.
Six 1.5 mL plastic micro centrifuge tubes were labeled with the same labels as the 6-well plate (Figure 1). The labels were written in a permanent ink marker. They were opened and placed on a plastic tube holder. 500 μL of trypsin was added to each tube. A timer was set for 4 minutes and nothing was added or removed for the entirety of the 4 minutes. 500 μL of cell culture media. Since the cells are located on the bottom of the wells, the pipette tip was used to scrape the bottom in order to receive most of the cells for a more accurate analysis. Likewise, the adjustable volume pipette was used to pick up the liquid in each well and release it to rinse the well, also to receive most of the cells for more accurate results. The mixture in each of the wells in the 6-well plate was transferred to their corresponding 1.5 mL tubes. Then, the six 1.5 mL tubes were placed in the centrifuge. When one tube was placed, another was placed directly opposite of it to ensure that the centrifuge would be balanced. They were centrifuged for three minutes. After taking the 1.5 mL tubes out of the centrifuge, the cell looked like a pellet at the bottom. Using a variable adjustable pipette, 1,000 μL of media was removed from each tube. During this step, it was important to avoid touching the cell pellet at the bottom. 50 μL of lysis buffer was added to each tube. Then, each 1.5 μL tube was vortexed for 10 seconds each using the Scientific Industries Vortex-Genie 1. Then, they were transferred to another plastic tube holder and kept in the fridge for 25 hours at -20°C.
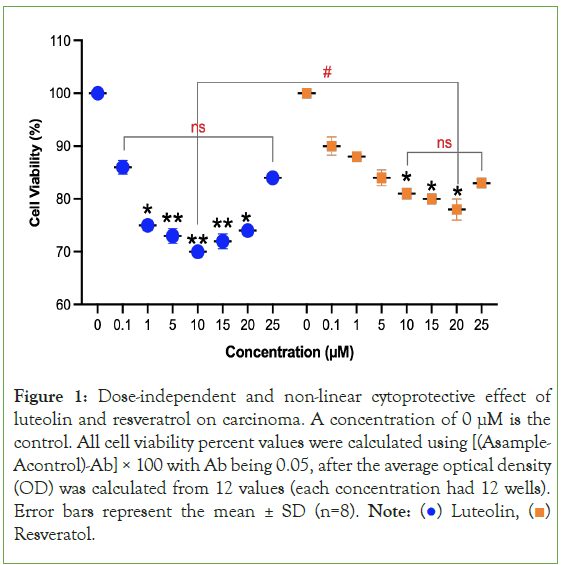
Figure 1: Dose-independent and non-linear cytoprotective effect of
luteolin and resveratrol on carcinoma. A concentration of 0 μM is the
control. All cell viability percent values were calculated using [(Asample-
Acontrol)-Ab] × 100 with Ab being 0.05, after the average optical density
(OD) was calculated from 12 values (each concentration had 12 wells).
Error bars represent the mean ± SD (n=8). Note:  Resveratol.
Resveratol.
A 96-well plate was used. One row on this 96-well plate has 12 wells and 2 wells were utilized per 1.5 mL tube. 50 μL of caspase assay buffer was added to each well. Then, 45 μL of lysis buffer was added to each well. 5 μL of the solution in each 1.5 mL was added to each well. Then, the BioVia microplate reader was set to a wavelength of 415. Results were taken in 0 minutes. A timer was set for 15 minutes, 30 minutes, 45 minutes, and 60 minutes so that for every 15 minute interval, results would be recorded.
ELISA assays were used to measure proteins of interest. After the cell cultures were seeded into each of 24 wells on a plate, 5 μL of luteolin, resveratrol, and a combination of both were added to 22 of the wells in various concentrations, and the remaining 2 wells were designated as the control group. Following an incubation period of one week, 500 μL of Phosphate Buffered Saline was added to each well. Afterwards, the cells were subjected to a -20°C environment for 30 minutes, and then taken out to thaw at 20°C for 30 minutes. In total, this cycle of freezing and thawing was done four times, in order to lyse the cells and release proteins normally found inside them. The samples from the wells were collected and placed into 7 distinct tubes. Then, the mitochondrial complex 1 and CHL-1 levels were measured using the standard “sandwich” enzyme-linked immunosorbent assay procedure according to the manufacturer’s protocol. A standard curve for each protein was collected and the protein levels were documented using the curve.
Experiment 2b: Improving luteolin and resveratrol’s therapeutic window
After methylating and glycosylating luteolin and resveratrol following standard procedures by Sigma Aldrich, they were left to rest for 24 hours before preparing them into treatments in the same way described in Experiment 1.
Results and Discussion
Experiment 1: The effect of luteolin on carcinoma cells; the effect of resveratrol on carcinoma cells
The behavior of luteolin and resveratrol demonstrate that there is an ideal concentration range where the treatments are the most cytoprotective. This can be viewed as the equivalent of a drug’s medicinal window. As shown in Figure 1, for luteolin, cancer cell death was induced the greatest at a molar concentration of 10 μM at 38.2%. For resveratrol, cancer cell death was induced the greatest at a molar concentration of 15 μM at 29.5%. In terms of comparing the two secondary metabolites, luteolin is more impactful in decreasing the toxicity of both types of cancer cells. As shown in Figure 1, when comparing the concentration of luteolin that was the most impactful to the concentration of resveratrol that was the most impactful on carcinoma cells, there was a significant difference (p=0.042) between them with luteolin decreasing carcinoma cell survival by 38.2% and resveratrol decreasing carcinoma cell survival by 29.5%. The relationship between concentration and the extent of cytoprotective effects is not a linear relationship and a higher dosage does not cause greater cytoprotection.
Both luteolin and resveratrol were able to remove colonies of carcinoma cells that have grown through pretreating and preparing the carcinoma cell line. Out of all the treatments, both luteolin and resveratrol removed the most amounts of carcinoma cell colonies at 10 μM. At each compound’s most effective concentration, luteolin removed more colonies than resveratrol. However, at lower concentrations before 10 μM, resveratrol consistently removed more colonies than luteolin. Both secondary metabolites removed less colonies at 15 μM and 20 μM compared to 10 μM. In fact, there was no statistically significant difference between the amount of colonies resveratrol removed at 5 μM and the amount of colonies resveratrol removed at 15 μM and 20 μM. This makes sense and is seen in Figure 2, where resveratrol’s point at 5 μM, 15 μM, and 20 μM are all at a similar point around 140 colonies.
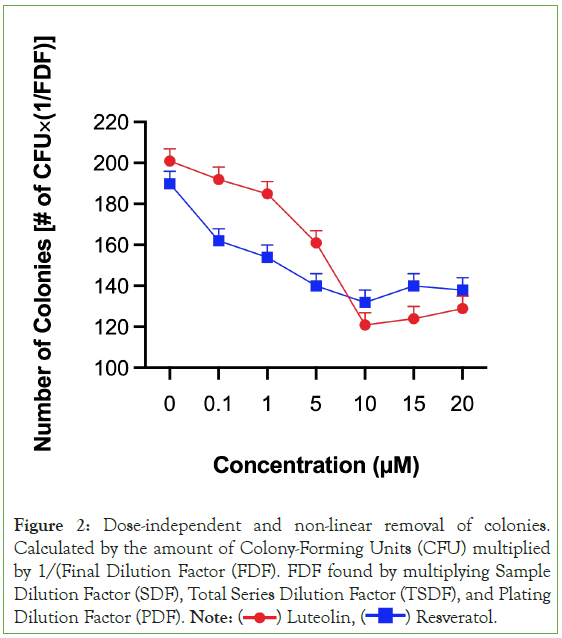
Figure 2: Dose-independent and non-linear removal of colonies.
Calculated by the amount of Colony-Forming Units (CFU) multiplied
by 1/(Final Dilution Factor (FDF). FDF found by multiplying Sample
Dilution Factor (SDF), Total Series Dilution Factor (TSDF), and Plating
Dilution Factor (PDF). Note:  Resveratol.
Resveratol.
The rates of migration is consistent with past studies on secondary metabolites such as flavonoids in that as the time that treatment is applied to the in vitro model, the greater the rate of migration. Luteolin behaved less in a time-dependent manner as at 15 μM and 20 μM, the treatment that was given 48 hours to rest actually inhibited migration at a lower rate than the treatment given 24 hours to rest (Figure 3). This can be seen with the orange bar (48 hours) being slightly higher than the green bay (24 hours). Resveratrol behaved in a fully time-dependent manner as for all treatments, the treatment left to rest for 48 hours surpassed the treatment left to rest for 24 hours in inhibiting migration Likewise, the treatment left to rest for 72 hours surpassed the treatment left to rest for 48 hours in inhibiting migration. The results as a whole indicate that luteolin and resveratrol have a strong therapeutic effect towards general carcinoma cells. Nevertheless, in all cases tested, the secondary metabolites resemble drugs in their limited therapeutic window. This is particularly interesting as past computational studies have indicated that compounds naturally occurring in plants are safe to use, even in abnormally large amounts [32]. However, the present study uniquely identifies that while luteolin and resveratrol do occur naturally in plants; they still decline in therapeutic efficacy as greater amounts are used. This implicates the potential adverse effects that luteolin and resveratrol derived dietary supplements may cause. Since 80% of supplement bottles do not contain the maximum dosage nor the dosage amount of each ingredient, the growing number of cancer patients who use luteolin and resveratrol derived supplements do not know how many capsules they are actually supposed to take. Plus, as more than 75% of Americans believe that mega dosing produces greater health benefits than taking a “normal dose” [33] the excessive intake of luteolin and resveratrol can potentially be interfering with the patient’s cancer treatment.
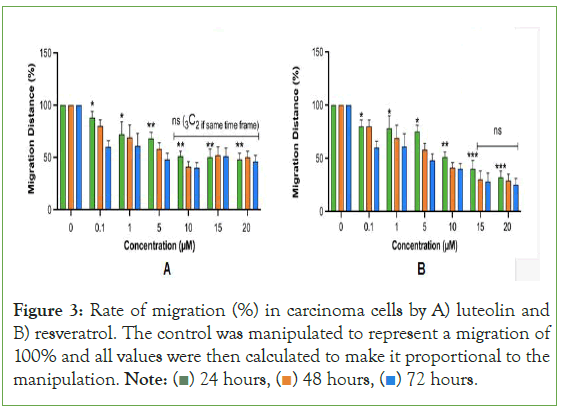
Figure 3: Rate of migration (%) in carcinoma cells by A) luteolin and
B) resveratrol. The control was manipulated to represent a migration of
100% and all values were then calculated to make it proportional to the
manipulation. Note:  72 hours.
72 hours.
The parallels between the two secondary metabolites and drugs include the fact that for both, the risk of being ineffective is at low concentrations and at high concentrations; the risk of adverse effects is increased. This is why physicians give patients dose regimens-designed to use the ideal therapeutic window in order to maximize efficacy and minimize side effects. However, with over- the-counter supplements, no physician is giving a patient a dose regimen. Luteolin and resveratrol used alone are likely not safe treatments for cancer.
There are two reasons why luteolin and resveratrol should be tested with a chemotherapy drug. First, past studies have indicated that combining two drugs, or combination therapy; lengthen the therapeutic window [34]. Another reason is that past research has clarified the potential of luteolin and resveratrol to decrease the unwanted cardiotoxicity that doxorubicin causes. One piece of evidence that supports the idea that the secondary metabolites luteolin and/or resveratrol may have this ability is that they are strong antioxidants. Studies have shown that doxorubicin causes myocardial damage by blocking the mechanism of antioxidant cells which in turn causes the accumulation of reactive oxygen species and increases the apoptosis of myocardial cells. Additionally, luteolin and resveratrol have been shown to exert a protective effect in heart injury models by protecting heart tissues in diabetic mice by modulating Nrf2-mediated oxidative stress and NF-κB-mediated inflammatory responses [25].
Experiment 2a: Luteolin and resveratrol reducing cardiotoxicity (an unwanted side effect of doxorubicin) while preserving doxorubicin’s cytotoxicity in carcinoma cells
Figure 4 demonstrates that when the in vitro cardiotoxicity model using cardiomyocytes are treated with doxorubicin, luteolin, and resveratrol, the beat rate as a percentage of the solvent control (DMSO), is significantly more stable and does not rise as much compared to when doxorubicin is the only chemical used on cardiomyocytes. This can be seen in the relatively straighter orange line compared to the blue line which is constantly changing in beat rate between treatments. However, luteolin and resveratrol still did increase the beat rate, just not as drastically (20 μM to 35 μM: 115.2%, 118.3%, 119.1%, 120.7%).
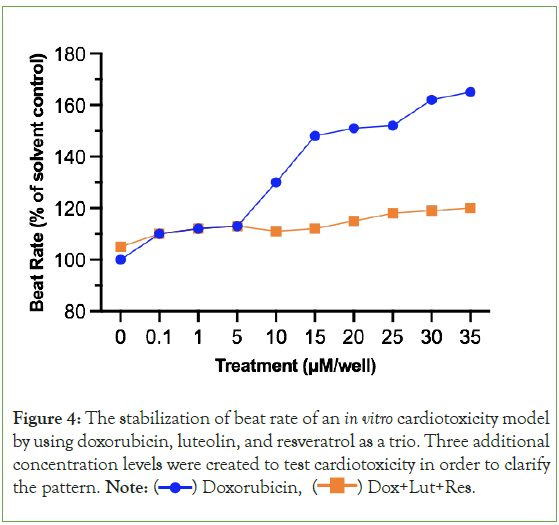
Figure 4: The stabilization of beat rate of an in vitro cardiotoxicity model
by using doxorubicin, luteolin, and resveratrol as a trio. Three additional
concentration levels were created to test cardiotoxicity in order to clarify
the pattern. Note:  Dox+Lut+Res.
Dox+Lut+Res.
A reduction of cardiotoxicity is not as valuable unless it is paired with the ability to preserve doxorubicin’s cytotoxic effects on cancer cells. Figure 5 demonstrates that luteolin and resveratrol are capable of preserving doxorubicin’s anticancer property of cytotoxicity. In fact, luteolin and resveratrol improves doxrubicin’s cytotoxicity, as seen in the orange line being lower than the blue line from 0.1 μM to 10 μM. One difference in the dose-response curves of doxorubicin compared to the two secondary metabolites is that doxorubicin’s progression in cytotoxicity ends and becomes relatively stable (straight line from 15 μM to 25 μM). Whereas, a recurring pattern in the present study is that luteolin and resveratrol’s progression in cytotoxicity does not become stable. Rather, it increases after the end of the therapeutic window (shown in the upward slope of the orange line after 10 μM).
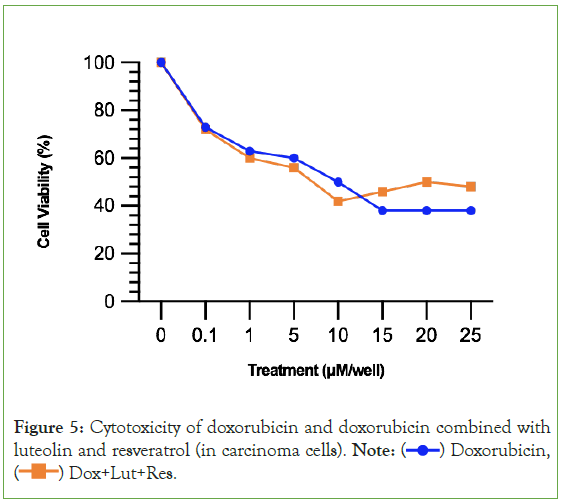
Figure 5: Cytotoxicity of doxorubicin and doxorubicin combined with
luteolin and resveratrol (in carcinoma cells). Note:  Doxorubicin,
Doxorubicin,  Dox+Lut+Res.
Dox+Lut+Res.
The KD’s reveal that luteolin strongly interacts with Caspase-3 Caspase-6, and Mitochondrial Complex 1. The KD’s reveal that resveratrol strongly interacts with the family of peroxisome proliferator-activated receptors (PPARɑ, PPAR-γ, and PPARδ) and Mitochondrial Complex 1. These strong interactions are indicated by the dark colors for them in Figure 6. The KDs also indicate that flavonoids interact with similar biological pathways and that stilbenoids interact with similar biological pathways. Quercetin and isorhamnetin are both flavonoids and their KDs mimic the KDs of luteolin. For instance, quercetin and isorhamnetin also interact strongly with Caspase-3, Caspase-6, and Mitochondrial Complex 1-just like luteolin. Piceatamnol and astringin also interact strongly with the family of peroxisome proliferator-activated receptors and Mitochondrial Complex 1. The revelation that luteolin interacts strongly with the same pathways as other flavonoids and resveratrol interacts strongly with the same pathways as other stilbenoids supports the notion that flavonoids are similar and can some findings about one type of flavonoid or stilbenoid can be generalized to other flavonoids and stilbenoids with similar structure.
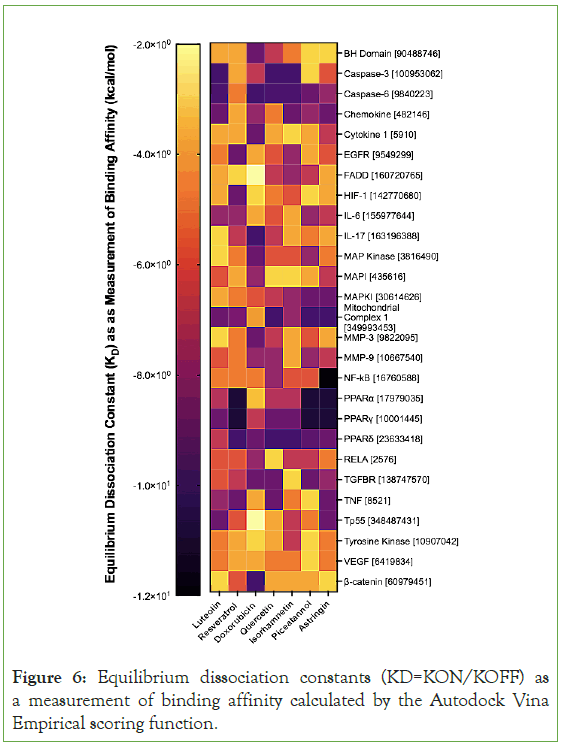
Figure 6: Equilibrium dissociation constants (KD=KON/KOFF) as a measurement of binding affinity calculated by the Autodock Vina Empirical scoring function.
The interactions that Autodock Vina computed were confirmed by western blot tests to measure proteins of interest. Like what Figure 7 confirms, luteolin and resveratrol both have high activity levels of Mitochondrial Complex 1, luteolin has high levels of various caspase proteins, and resveratrol has high levels of the peroxisome proliferator-activated receptors. Besides the high level of certain biological pathway activity, the western blots also indicate that these pathways are likely to be explanations of the therapeutic effects of the secondary metabolites. In Experiment 1, the treatments of 5 μM and 10 μM are consistently the treatments that had had the greatest cytotoxicity to carcinoma cells and reduced migration and adhesion the most. One explanation for the lower activity of all three of the peroxisome proliferator-activated receptors for resveratrol is that rather than activating the receptors, resveratrol inhibits the receptors. This would explain why the 10 μM treatment had the lowest activity level for the receptors yet had was the same treatment that produced the greatest reduction of cytotoxicity.
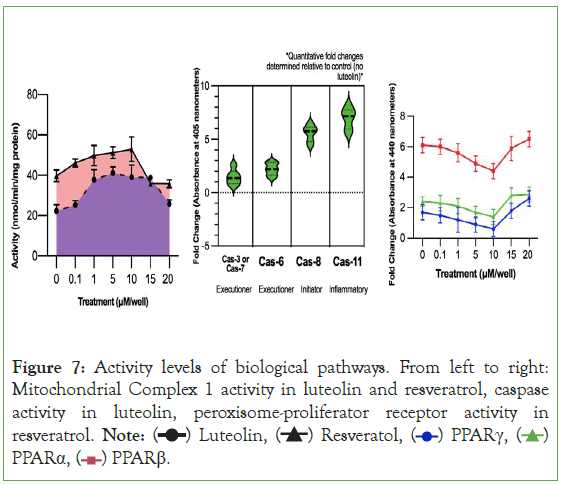
Figure 7: Activity levels of biological pathways. From left to right:
Mitochondrial Complex 1 activity in luteolin and resveratrol, caspase
activity in luteolin, peroxisome-proliferator receptor activity in
resveratrol. Note: PPARα,
PPARα,  PPARβ.
PPARβ.
However, what this means in context of the present study is that there likely will not be a single secondary metabolite that does not have some type of non-linearity. In order to truly improve doxorubicin-based chemotherapy treatment, the compounds added to the treatment ideally have to be dose-dependent, with the higher doses inducing greater cancer death and reducing metastasis the most.
The results of luteolin and resveratrol are promising but clarify a downside to using them. With the results of luteolin and resveratrol alone, the therapeutic effects of them individually are confined to a limited range. While luteolin and resveratrol were effective at concentrations generally up to 15 μM and did avoid excessive cardiotoxicity caused by doxorubicin-chemotherapy delivery, these two secondary metabolites were inconsistent and therefore are not the best solution to improving doxorubicin- chemotherapy delivery. Additionally, from the results in Figure 6, many secondary metabolites and natural compounds similar to luteolin and resveratrol have the same mechanisms of action which reasonably indicates that they likely will act in a similar manner. In Figure 7, the fact that none of the activity levels of the biological pathways were similar in fold change during the treatments of 10 μM, 15 μM, or 20 μM further supports the interpretation that the secondary metabolites have a limited therapeutic window.
Researchers have been concerned about the clinical utilization of secondary metabolites and natural compounds because of their low yield when extracted from plants and less than optimal Pharmacokinetics (PK) profile-Absorption, Distribution, Metabolism, and Excretion (ADME) [35]. If a biosynthetically engineered compound is created, it would relieve the problem about the difficulty of extracting luteolin and resveratrol (they are present in very minute quantities per kilogram of plant biomass). If luteolin and resveratrol are continuously extracted, the agriculture sector would be greatly harmed. Thus, if a compound is synthetically made, then the hazards of isolating a compound of interest in crude mixture would be alleviated.
In terms of alleviating the problem with luteolin and resveratrol’s unideal PK profile, there are two revisions that past research indicates may help. One way is by increasing methylation (addition of a methyl group). O-methylated secondary metabolites have better bioavailability due to better absorption and increased permeability [36]. The improved metabolic stability is caused by reducing the conjugation reaction by glucuronidation and sulfation-two aspects that are partially responsible for poor bioavailability and stability of secondary metabolites [37]. Another way is to increase glycosylation by adding a sugar moiety through a hydroxyl bond. Glycosylation helps compounds become more water-soluble and stable [38]. These two methods could increase the therapeutic window of the two secondary metabolites.
Experiment 2b: Improving luteolin and resveratrol’s therapeutic window
By simply methylating and glycosylating luteolin and resveratrol, then combining them together, 1-(2,4,6-trihydroxyphenyl)-5,7- dihydroxy-2H-1-benzene-1,3-diol (TDB-13) solves the weakness observed in Experiment 2A where luteolin and resveratrol had a small therapeutic window [39-41]. In Figure 8, TDB-13 reduced cardiotoxicity more than luteolin and resveratrol. It is not consistently rising as seen in the small increase then decrease of the green line increasing after 15 μM. In Figure 9, the improvement in the therapeutic window is seen more clearly. Compared to Figures 5 and 9 demonstrates that TDB-13 mimics doxorubicin’s therapeutic window in that after a certain concentration, rather than declining in cytotoxicity, the treatments become stable and the line does not decrease or increase.
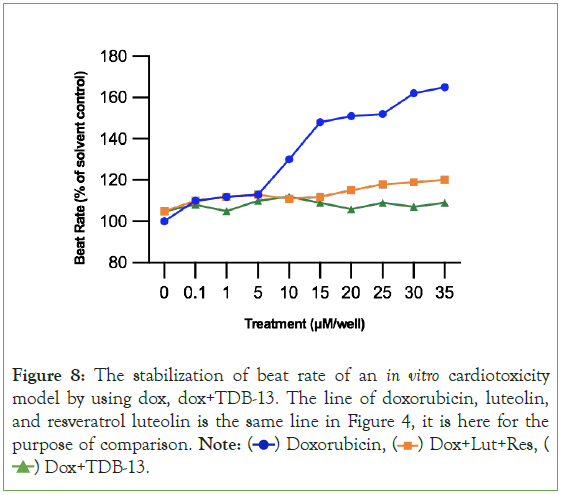
Figure 8: The stabilization of beat rate of an in vitro cardiotoxicity
model by using dox, dox+TDB-13. The line of doxorubicin, luteolin,
and resveratrol luteolin is the same line in Figure 4, it is here for the
purpose of comparison. Note:  Dox+Lut+Res,
Dox+Lut+Res,  Dox+TDB-13.
Dox+TDB-13.
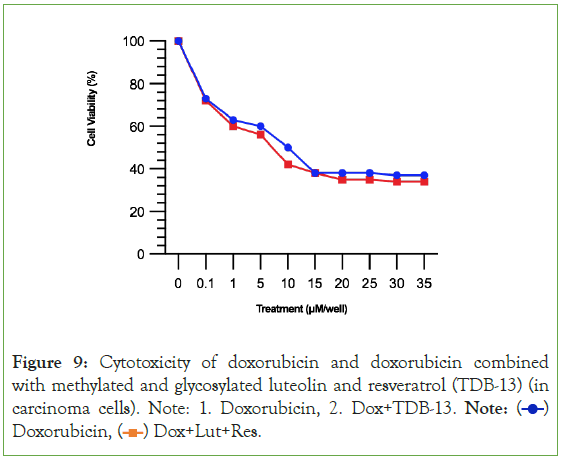
Figure 9: Cytotoxicity of doxorubicin and doxorubicin combined
with methylated and glycosylated luteolin and resveratrol (TDB-13) (in
carcinoma cells). Note: 1. Doxorubicin, 2. Dox+TDB-13. Note:  Doxorubicin,
Doxorubicin,  Dox+Lut+Res.
Dox+Lut+Res.
These results indicate that by changing a few properties of luteolin and resveratrol, they can become a more viable solution to improving doxorubicin-chemotherapy treatment.
Conclusion
Widely believed by cancer patients and the general public to have healing properties, the present study demonstrates a weakness of luteolin and resveratrol. Unlike drugs like doxorubicin, luteolin and resveratrol do not behave the same as a drug would. Uniquely, luteolin and resveratrol’s therapeutic window is not characterized by dose-response curves that end with an unchanging response (horizontal line) but rather a continually changing response that reduces its therapeutic effect. The implications of this are present in the dietary supplements derived from luteolin and resveratrol that a growing number of cancer patients use. If the results are translatable in humans, cancer patient’s having more luteolin and resveratrol in their diet could be detrimental to their treatment. This information can be encouragement to use dietary supplements with caution and to spread awareness about the risks of self-prescribing over-the-counter products.
Though not ideal to use on their own, luteolin and resveratrol are capable of improving chemotherapy through improving doxorubicin. The present study uniquely identified the therapeutic window of luteolin and resveratrol that is consistent through all the in vitro experiments. A simple solution to the limited therapeutic window is by methylating and glycosylating luteolin and resveratrol, and then combining them together. By being able to maintain and even improve doxorubicin’s toxicity towards carcinoma cells while decreasing cardiotoxicity, the two secondary metabolites may be a viable solution to improve chemotherapy.
The biggest limitation to the present study is that all experiments were done using in vitro models. Therefore, many clinical trials may be done in the future to fully integrate luteolin and resveratrol into chemotherapy treatment. In the past, some results of research done in vitro were not represented in the research done in vivo. Cancer in humans is very different from patient-derived cancer cells. Therefore, some scientists believe that animals must be used in research. However, some scientists believe that only 6% of animal studies can actually be translated to humans and those in vitro studies are more viable.
References
- Liu YQ, Wang XL, He DH, Cheng YX. Protection against chemotherapy-and radiotherapy-induced side effects: A review based on the mechanisms and therapeutic opportunities of phytochemicals. Phytomedicine. 2021;80:153402.
[Crossref] [Google Scholar] [PubMed]
- Ferrucci LM, McCorkle R, Smith T, Stein KD, Cartmel B. Factors related to the use of dietary supplements by cancer survivors. J Altern Complement Med. 2009;15(6):673-680.
[Crossref] [Google Scholar] [PubMed]
- Skibola CF, Smith MT. Potential health impacts of excessive flavonoid intake. Free Radic Biol Med. 2000;29(3-4):375-383.
[Crossref] [Google Scholar] [PubMed]
- Galati G, O'brien PJ. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37(3):287-303.
[Crossref] [Google Scholar] [PubMed]
- Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020;12(2):457.
[Crossref] [Google Scholar] [PubMed]
- O’Neill CB, Atoria CL, O’Reilly EM, Henman MC, Bach PB, Elkin EB. ReCAP: Hospitalizations in older adults with advanced cancer: The role of chemotherapy. J Oncol Pract. 2016;12(2):151-2.
[Crossref] [Google Scholar] [PubMed]
- Maeda H, Khatami M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin Transl Med. 2018;7(1):1-20.
[Crossref] [Google Scholar] [PubMed]
- Huang CY, Ju DT, Chang CF, Reddy PM, Velmurugan BK. A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer. Biomedicine. 2017;7(4).
[Crossref] [Google Scholar] [PubMed]
- Podyacheva EY, Kushnareva EA, Karpov AA, Toropova YG. Analysis of models of doxorubicin-induced cardiomyopathy in rats and mice. A modern view from the perspective of the pathophysiologist and the clinician. Front Pharmacol. 2021;12:670479.
[Crossref] [Google Scholar] [PubMed]
- Liu Q, Luo X, Yi L, Zeng X, Tan C. First-line chemo-immunotherapy for extensive-stage small-cell lung cancer: A united states-based cost-effectiveness analysis. Front Oncol. 2021;11:699781.
[Crossref] [Google Scholar] [PubMed]
- Squamous cell carcinoma overview. Moffitt Cancer Center. (2022).
- McDermott M, Eustace AJ, Busschots S, Breen L, Crown J, Clynes M, et al. In vitro development of chemotherapy and targeted therapy drug-resistant cancer cell lines: A practical guide with case studies. Front Oncol. 2014;4:40.
[Crossref] [Google Scholar] [PubMed]
- Ajaykumar C. Overview on the side effects of Doxorubicin. Adv Precis Med Oncol. 2020.
- Howard GR, Jost TA, Yankeelov TE, Brock A. Quantification of long-term doxorubicin response dynamics in breast cancer cell lines to direct treatment schedules. PLoS Comput Biol. 2022;18(3):1009104.
[Crossref] [Google Scholar] [PubMed]
- Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E, et al. Mortality due to cancer treatment delay: Systematic review and meta-analysis. Brit Med J. 2020;371.
[Crossref] [Google Scholar] [PubMed]
- P, Abenojar E, De Leon A, Wegierak D, Exner AA. Increasing doxorubicin loading in lipid-shelled perfluoropropane nanobubbles via a simple deprotonation strategy. Front Pharmacol. 2020;11:644.
[Crossref] [Google Scholar] [PubMed]
- Argenziano M, Gigliotti CL, Clemente N, Boggio E, Ferrara B, Trotta F, et al. Improvement in the anti-tumor efficacy of doxorubicin nanosponges in in vitro and in mice bearing breast tumor models. Cancers. 2020;12(1):162.
[Crossref] [Google Scholar] [PubMed]
- Stewart DJ, Stewart AA, Wheatley-Price P, Batist G, Kantarjian HM, Schiller J, et al. The importance of greater speed in drug development for advanced malignancies. Cancer Med. 2018;7(5):1824-1836.
[Crossref] [Google Scholar] [PubMed]
- Shaikh F, Dupuis LL, Alexander S, Gupta A, Mertens L, Nathan PC. Cardioprotection and second malignant neoplasms associated with dexrazoxane in children receiving anthracycline chemotherapy: A systematic review and meta-analysis. J Natl Cancer Inst. 2016;108(4).
[Crossref] [Google Scholar] [PubMed]
- Alam M, Bano N, Ahmad T, Sharangi AB, Upadhyay TK, Alraey Y, et al. Synergistic role of plant extracts and essential oils against multidrug resistance and gram-negative bacterial strains producing extended-spectrum β-lactamases. Antibiotics. 2022;11(7):855.
[Crossref] [Google Scholar] [PubMed]
- Park HK, Lee JE, Lim J, Jo DE, Park SA, Suh PG, et al. Combination treatment with doxorubicin and gamitrinib synergistically augments anticancer activity through enhanced activation of Bim. BMC Cancer. 2014;14(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Maadi H, Soheilifar MH, Choi WS, Moshtaghian A, Wang Z. Trastuzumab mechanism of action; 20 years of research to unravel a dilemma. Cancers. 2021;13(14):3540.
[Crossref] [Google Scholar] [PubMed]
- Christodoulou C, Kostopoulos I, Kalofonos HP, Lianos E, Bobos M, Briasoulis E, et al. Trastuzumab combined with pegylated liposomal doxorubicin in patients with metastatic breast cancer. Oncol. 2009;76(4):275-285.
[Crossref] [Google Scholar] [PubMed]
- Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Luo Y, Shang P, Li D. Luteolin: A flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front Pharmacol 2017;8:692.
[Crossref] [Google Scholar] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin. 2021;71(1):7-33.
[Crossref] [Google Scholar] [PubMed]
- Pijuan J, Barceló C, Moreno DF, Maiques O, Sisó P, Marti RM, et al. In vitro cell migration, invasion, and adhesion assays: from cell imaging to data analysis. Front Cell Dev Biol. 2019;7:107.
[Crossref] [Google Scholar] [PubMed]
- Friedl P, Alexander S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell. 2011;147(5):992-1009.
[Crossref] [Google Scholar] [PubMed]
- Johnson-Arbor K, Dubey R. Doxorubicin. StatPearls Publishing. 2022.
- Bandyopadhyay A, Wang L, Agyin J, Tang Y, Lin S, Yeh IT, et al. Doxorubicin in combination with a small TGFβ inhibitor: A potential novel therapy for metastatic breast cancer in mouse models. PLoS One. 2010;5(4):10365.
[Crossref] [Google Scholar] [PubMed]
- Zhang Q, Khetan A, Er S. Comparison of computational chemistry methods for the discovery of quinone-based electroactive compounds for energy storage. Sci Rep. 2020;10(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Starr RR. Too little, too late: Ineffective regulation of dietary supplements in the United States. Am J Public Health. 2015;105(3):478-485.
[Crossref] [Google Scholar] [PubMed]
- Goldstein LJ, Mansutti M, Levy C, Chang JC, Henry S, Fernandez-Perez I, et al. A randomized, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (fRida). Breast Cancer Res Treat. 2021;190(2):265-75.
[Crossref] [Google Scholar] [PubMed]
- Bitew M, Desalegn T, Demissie TB, Belayneh A, Endale M, Eswaramoorthy R. Pharmacokinetics and drug-likeness of antidiabetic flavonoids: Molecular docking and DFT study. Plos One. 2021 Dec 10;16(12):e0260853.
[Crossref] [Google Scholar] [PubMed]
- Sajid M, Channakesavula CN, Stone SR, Kaur P. Synthetic biology towards improved flavonoid pharmacokinetics. Biomolecules. 2021;11(5):754.
[Crossref] [Google Scholar] [PubMed]
- Khodzhaieva RS, Gladkov ES, Kyrychenko A, Roshal AD. Progress and achievements in glycosylation of flavonoids. Front Chem. 2021;9:637994.
[Crossref] [Google Scholar] [PubMed]
- Harvie M. Nutritional supplements and cancer: Potential benefits and proven harms. Am Soc Clin Oncol Educ Book. 2014;34(1):478-486.
[Crossref] [Google Scholar] [PubMed]
- ACS Medical Content and News Staff. Cancer facts and figures. American Cancer Society. 2022.
[Crossref]
- Fania L, Didona D, Di Pietro FR, Verkhovskaia S, Morese R, Paolino G, et al. Cutaneous squamous cell carcinoma: From pathophysiology to novel therapeutic approaches. Biomedicines. 2021;9(2):171.
[Crossref] [Google Scholar] [PubMed]
- MTT assay protocol for cell viability and proliferation. Sigma Aldrich. 2018.
- Yogev O, Almeida GS, Barker KT, George SL, Kwok C, Campbell J, et al. In Vivo modeling of chemoresistant neuroblastoma provides new insights into chemorefractory disease and metastasismodeling chemoresistance and metastasis in neuroblastoma. Cancer research. 2019;79(20):5382-5393.
[Crossref] [Google Scholar] [PubMed]
Citation: Dong J (2023) Improving Doxorubicin-Chemotherapy Treatment with Luteolin and Resveratrol: A Novel Synthetically Engineered Secondary Metabolite “TDB-13” Created with Luteolin and Resveratrol. J Biol Res Ther. 12:260.
Copyright: © 2023 Dong J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

