Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 1
Identification of Occult Hepatitis B Infection (OBI) among Blood Donors at Aseer Southern Region in Saudi Arabia
Asra Khanum MD1, Ahmad M Alamri PhD2, Abdurrahman M Alshehri1, Abdulaziz S Alsabrah1, Mohammed S Alshehri1, Galib M Albahja1 and Fahad S Alshehri PhD3,4*2Department of Clinical Laboratory Science, King Khalid University, Abha, Saudi Arabia
3Department of Pathology and Clinical Laboratory Medicine, Haematology Section, King Faisal Medical City for Southern Region, Abha, Saudi Arabia
4Department of Pathology and Clinical Laboratory Medicine, Haematology Section, King Fahad Medical City, Riyadh, Saudi Arabia
Received: 11-Dec-2023, Manuscript No. JBDT-23-24295; Editor assigned: 13-Dec-2023, Pre QC No. JBDT-23-24295 (PQ); Reviewed: 03-Jan-2024, QC No. JBDT-23-24295; Revised: 10-Jan-2024, Manuscript No. JBDT-23-24295 (R); Published: 17-Jan-2024, DOI: 10.4172/2155-9864.24.15.572
Abstract
Background: Hepatitis B infection (HBV) and other transfusion-transmitted viruses remain a major concern in transfusion medicine. Blood transfusions can spread Occult HBV Infection (OBI), but the prevalence is limited. OBIs in donors have been obvious after blood transfusion organizations began using sensitive HBV Deoxyribonucleic acid (DNA) Nucleic Acid Amplification Technique (NAT) screening. Residual risk assessments may not accurately predict transfusion-related OBI infection rates. Several nations now use HBV DNA confirmatory Nucleic Acid Tests (NATs).
Materials and methods: In this study, we screened blood donors in the “Aseer” Province of Saudi Arabia for HBV DNA, anti-HBc, and HBV surface Antigen (HBsAg) to determine the prevalence and annual trends of OBI infection. The present study was conducted from January 2022 to December 2022.
Results and discussion: Total blood samples for donation in year 2022 from January to December were 28232 donors from 18 hospitals of “Aseer” Region. The total of HBs Ag positive samples was (133; 0.47), Anti HBc reactive samples were (908; 3.21%), only 16 donor’s samples were tested reactive for HBV DNA by NAT. The OBI cases detected from this population were only (16; 18%) and (6; 37.50%) were examined as seropositive OBI and (10; 62.50%) were detected as a seronegative OBI.
Conclusion: Based on our research findings, the prevalence of Occult Blood Infections (OBI) among blood donors in the “Aseer” region not a statistically significant however, we need to avoid the transmission of these limited OBI cases in the general population by strict regulation from the preventive medicine department in the region.
Keywords
Occult hepatitis B infection; Transfusion; Nucleic acid testing
Abbreviations
ACH: Aseer Central Hospital; AMCH: Abha Maternity and Children Hospital; KMMCH: Khamis Mushait Maternity and Children Hospital; KMGH: Khamis Mushayt General Hospital; MGH: Mahael General Hospital; MJGH: Majardah General Hospital; RAGH: Rijal Alma’a General Hospital; ARGH: Ahad Rufaidah General Hospital; SAGH: Sarat Abidah General Hospital; DGH: Dhahran Al Janoub General Hospital; NGH: Namas General Hospital; BGH: Billasmar General Hospital; QGH: Qahma General Hospital; BRGH: Birk General Hospital; PFKCC: Prince Faisal Bin Khalid Cardiac Center; HNH: Hayat National Hospital; SGH: Saudi Germany Hospital; APH: Abha Private Hospital; CBB: Central Blood Bank
Introduction
Hepatitis B is a prominent public health concern, with modifiable risk factors potentially exacerbating disease progression [1]. The Hepatitis B Virus (HBV) is a viral infection that targets the liver and has the potential to induce both acute and chronic illnesses [2]. The global prevalence of chronic hepatitis B virus (HBV) infection, commonly referred to as CHB, was estimated to be 4.1% in the year 2019. This number corresponds to a total of 316 million individuals worldwide who were living with HBV [3]. The progression of chronic viral hepatitis can lead to severe consequences, including cirrhosis and hepatocellular cancer, which pose significant risks to an individual's life. Based on variations in life expectancy, it has been observed that a proportion exceeding 20% of individuals afflicted with chronic infection progress to end- stage diseases. Consequently, this unfortunate outcome contributed to a total of 555,000 fatalities worldwide in the year 2019 [3]. Hepatitis B virus (HBV) infection has the potential to elicit a diverse range of clinical manifestations, spanning from a state of inactive carrier to severe forms of hepatitis, cirrhosis, or hepatocellular cancer [4].
A significant proportion of individuals may harbour the Hepatitis B Virus (HBV) for an extended period following the resolution of acute infection, without exhibiting any discernible signs of liver disease [5]. Serological markers can be employed to distinguish various clinical stages of viral persistence. The diagnosis of HBV infection often occurs upon the detection of circulating hepatitis B surface Antigen (HBsAg) [6]. Chronic infection is distinguished by the enduring presence of the antigen and the detection of HBV DNA in the serum. Conversely, resolved HBV infection is indicated by the presence of seropositivity for–Hepatitis B core (HBc) and Hepatitis B surface (HBs) antibodies in patients [7].
The act of blood donation involves the extraction of blood from a medically fit individual, followed by its collection and preservation for subsequent transfusion into a recipient who possesses a compatible blood type. The utilization of blood transfusion is significant in the preservation of patient lives and the stabilization of their medical conditions [8]. However, the transmission of diseases through transfusion, facilitated by blood-borne pathogens, continues to be a subject of medical apprehension [9]. The predominant transfusion-associated dangers on a global scale encompass Human Immunodeficiency Virus- Acquired Immunodeficiency Syndrome (HIV-AIDS) and hepatitis [10]. Hepatitis is a significant hepatic ailment resulting from a collection of viral agents. Hepatitis is distinguished by hepatic inflammation accompanied by the infiltration of inflammatory cells, which is instigated by an infection and has the potential to result in grave complications such as fibrosis or cirrhosis, ultimately posing a risk of fatality [11]. The primary etiology of hepatitis infections is attributed to a collection of five distinct viruses, specifically hepatitis viruses A, B, C, D, and E [12]. Nevertheless, the primary health problems are predominantly attributed to the Hepatitis B Virus (HBV) owing to its elevated infection rates, diverse routes of transmission, and early asymptomatic phase that hinders timely detection, hence leading to the chronic character of the disease [13].
Hepatitis B has the potential to be spread through both horizontal and vertical (perinatal) modes of transmission. The transmission of the infection takes place via several means, including contact with contaminated blood or blood products, engaging in sexual intercourse with an individual who is infected, or sharing needles [10]. Furthermore, it is important to note that an HBV-infected mother has the potential to transmit the virus to her offspring prior to, during, or after the process of childbirth [14]. The prevention of these infections is mostly dependent on the appropriate and timely administration of vaccines, as well as the serological screening of blood donors using Hepatitis B surface Antigen (HBsAg) testing for Hepatitis B Virus (HBV) [15]. Certain countries are utilizing anti-Hepatitis B core antigen Antibody (HBcAb) testing to identify individuals with chronic Hepatitis B Virus (HBV) infection who have a low viral load in their blood but do not exhibit detectable Hepatitis B surface Antigen (HBsAg) [16]. In numerous developed nations, including Saudi Arabia, the implementation of Nucleic Acid Amplification Testing (NAT) for HBV has proven to be effective in screening donors in recent years [17]. The prevalence of hepatitis viruses among the global population is estimated to affect around 2.3 billion individuals, leading to approximately 1.4 million fatalities [18]. Notably, hepatitis B accounts for 90% of these deaths. Hence, the prevalence of chronic hepatitis infections has led to a heightened death rate and presents a significant strain on healthcare systems in multiple nations, including Saudi Arabia [19]. Numerous studies have been undertaken in Saudi Arabia to examine the prevalence of Hepatitis B Virus (HBV) among individuals donating blood. However, there is a dearth of research focusing on the prevalence rates of HBV among blood donors residing in metropolitan areas within the country [20].
Occult Hepatitis B Infection (OBI) refers to the condition in which Hepatitis B Virus (HBV) DNA is present in the liver, regardless of whether HBV DNA is detectable or undetectable in the serum, among persons who have tested negative for Hepatitis B surface Antigen (HBsAg) [21]. In instances of genuine occult hepatitis B infection (OBI), the concentration of viral DNA in the serum is generally observed to be little. The diagnosis of Occult hepatitis B Infection (OBI) is frequently accomplished by employing blood HBV DNA and viral marker assays, as evaluating liver tissue may not always be feasible or viable [22]. The transmission of Hepatitis B Virus (HBV) through blood transfusion from a donor with occult HBV infection (OBI) was initially documented in 1978 [23]. There has been a recent surge in the publication of papers examining the infectivity of blood products in relation to OBI.
The objective of this retrospective study was to determine the prevalence of Occult Blood borne Infections (OBI) among blood donors in the Assir region of the Kingdom of Saudi Arabia (K.S.A.) over the period of January to December 2022.
Materials and Methods
The present study was conducted from January 2022 to December 2022, encompassing all individuals who participated as blood donors at the Blood Banks in the “Aseer” Region. Serum (serology) and plasma (NAT) samples were obtained from all donors and subjected to testing for Hepatitis B surface Antigen (HBsAg), antibodies against Hepatitis B core antigen (anti-HBc), and antibodies against Hepatitis B surface antigen (anti-HBs) using the Chemi Luminescent Immunoassay (CLIA) method on the Alinitys equipment. Additionally, all donors were screened for the presence of hepatitis B virus (HBV)-DNA using Nucleic Acid Testing (NAT). The blood donor samples were found to be negative for Hepatitis B surface Antigen (HBsAg) but positive for Hepatitis B virus (HBV) DNA, with or without the presence of Hepatitis B core Antibody (HBcAb) reactivity. These samples were classified as cases of occult Hepatitis B Infection (OBI). This study was approved from Asser Institutional Review Board (IRB) under number REC-04-09-2022 and date 09/12/2022 and all donors were signed the official consent form. GraphPad Prism 5.04 has been used for the purpose of data analysis.
Results
Total blood samples of donation in “Aseer” region
Total blood samples for donation in year 2022 from January to December were 28232 donors from 18 hospital of “Aseer” Region (Figure 1).
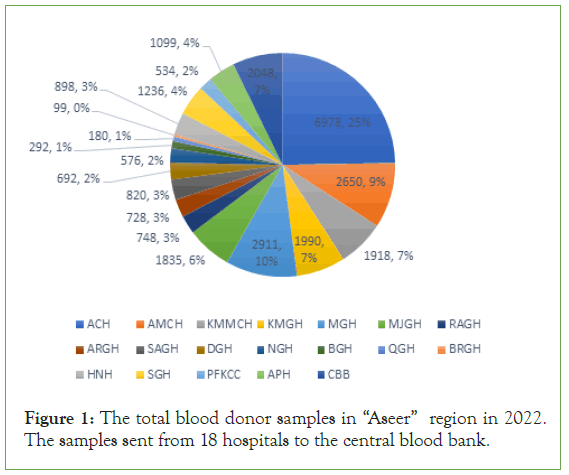
Figure 1: The total blood donor samples in “Aseer” region in 2022. The samples sent from 18 hospitals to the central blood bank.
The percentage of these blood samples were (6978; 25% ACH, 2650; 9% AMCH, 1918; 7% KMMCH, 1990; 7% KMGH, 2911; 10% MGH, 1835; 6% MJGH, 784; 3% RAGH, 728; 3% ARGH, 820; 3% SAGH, 692; 2% DGH, 576; 2% NGH, 292; 1% BGH, 180; 1% QGH, 99; 0% BRGH, 898; 3% HNH, 1236; 4% SGH, 534; 2% PFKCC, 1099; 4% APH and 2048; 7% CBB; Figure 1).
Detection of HBV reactive donor’s samples
Out of these blood donors, the total of HBs Ag positive samples were (133; 0.47) from the total blood donor samples. These numbers distributed between these hospitals’ (31; 23% ACH, 7; 5% AMCH, 6; 4% KMMCH, 5; 4% KMGH, 20; 15% MGH, 14; 11% MJGH, 4; 3% RAGH, 1; 1% ARGH, 7; 5% SAGH, 9; 7% DGH, 2; 1% NGH, 1; 1% NGH, 1; 1 APH and 25; 19% CBB) (Figure 2).
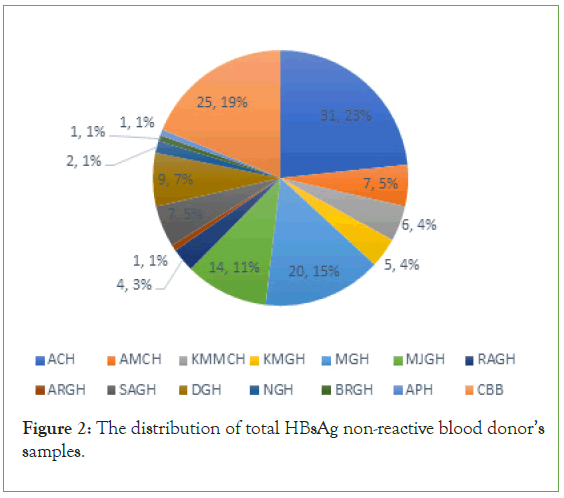
Figure 2: The distribution of total HBsAg non-reactive blood donor’s samples.
The total of Anti HBc reactive samples were (908; 3.21%) of total blood donor samples. It was from (317; 22% ACH, 94; 7% AMCH, 84; 6% KMMCH, 112; 8% KMGH, 167; 12% MGH, 136; 10% MJGH, 38; 3% RAGH, 28; 2% ARGH, 47; 3% SAGH, 47; 3% DGH, 25; 2% NGH, 8; 1% BGH, 4; 0% QGH, 7; 0% BRGH, 16; 1% PFKCC, 11; 1% APH and 273; 22% CBB) (Figure 3).
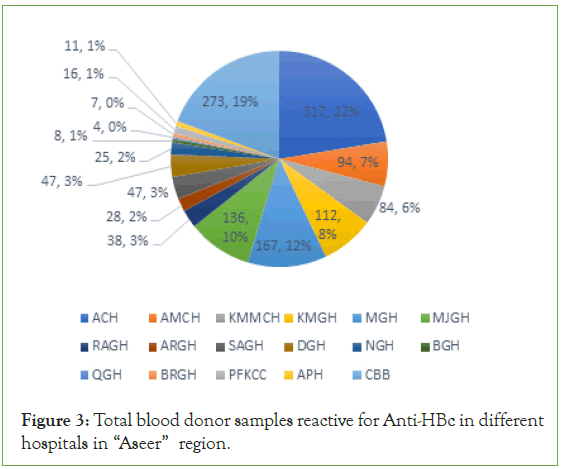
Figure 3: Total blood donor samples reactive for Anti-HBc in different hospitals in “Aseer” region.
The prevalence of OBI cases among blood donation in “Aseer” region
In contrast, only 16 donor’s samples were tested reactive for HBV DNA by NAT. These positive samples were (4; 25% ACH, 1; 6% AMCH, 1; 6% KMGH, 1; 6% MJGH, 1; 6% RAGH, 2; 13% DGH and 6; 38% CBB) (Figure 4).
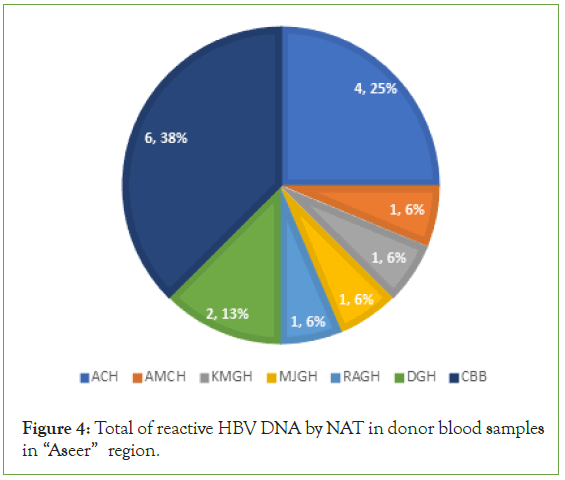
Figure 4: Total of reactive HBV DNA by NAT in donor blood samples in “Aseer” region.
Of the total of Hepatitis B positive cases only (85; 0.30%) samples confirm as reactive for HBsAg. After matching the markers of occult hepatitis B virus (OBI) from this population we found that, only (16; 18%) were a considered as OBI positive cases (Figure 5).
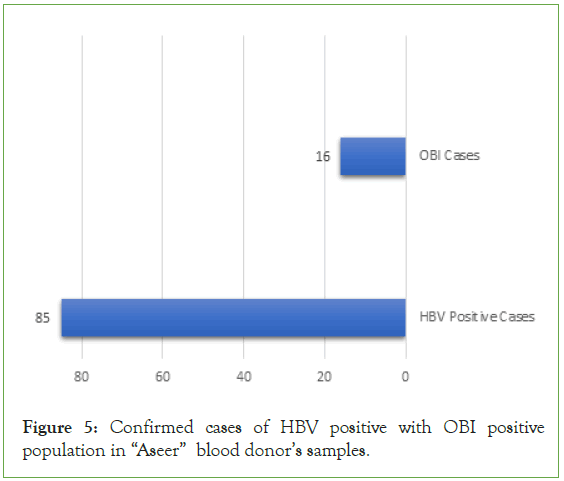
Figure 5: Confirmed cases of HBV positive with OBI positive population in “Aseer” blood donor’s samples.
According to the population of OBI, we detect the seropositive OBI which was non-reactive for HBsAg but reactive for Anti-HBc antibodies and reactive for HBV DNA and we found that, (6; 37.50%) were examined as seropositive OBI (Figure 6).
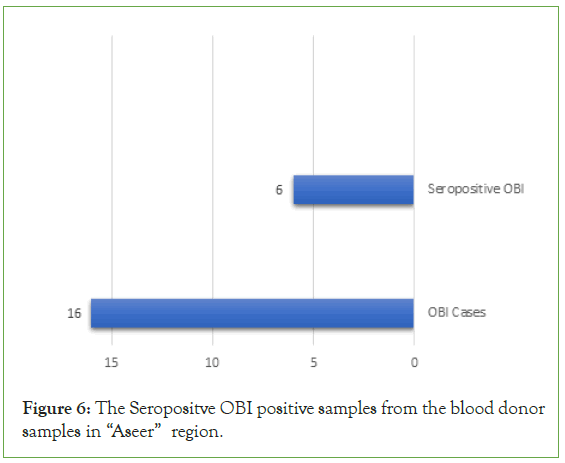
Figure 6: The Seropositve OBI positive samples from the blood donor samples in “Aseer” region.
In addition, the seronegative from the OBI population were identified which were a non-reactive for HBsAg and Anti-HBc Antibodies but reactive for HBV DNA by NAT test. We found that, (10; 62.50%) were detected as a seronegative OBI cases (Figure 7).
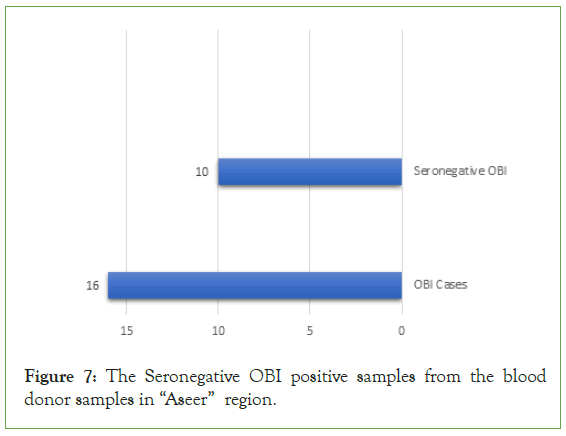
Figure 7: The Seronegative OBI positive samples from the blood donor samples in “Aseer” region.
Further, 823(2.91%) were HbsAg non-reactive but anti-HBc reactive, indicating chronic or resolving infection. HBV cases were increased significantly from over a period of time due to recent diagnostic technique where Occult Hepatitis infections OBI are diagnosed inspite of serology results non-reactive. OBI cases 16; 18% donors were diagnosed as HBV reactive were as HbsAg non-reactive only 6 cases Anti HBc reactive.
These results show that the prevalence of HBV occult hepatitis B infection among the blood donors. The central blood bank in “Aseer” region recognized this issue and need systematic pathway and more awareness to detect the future OBI cases (Figure 8).
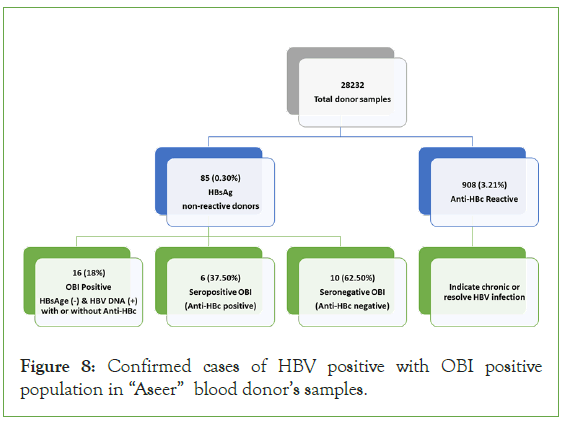
Figure 8: Confirmed cases of HBV positive with OBI positive population in “Aseer” blood donor’s samples.
Discussion
The danger of hepatitis B infection among blood donors and the donation process remains a significant concern in the field of transfusion medicine [24]. The World Health Organization (WHO) strongly recommends the implementation of mandatory screening tests for blood donors to mitigate the risk of transmission of this virus and other related diseases. The execution of these screening tests, particularly in low-income countries, will contribute to the World Health Organization's objective of eliminating the transmission of HBV infection through blood transfusion by 2030 [25].
The identification of occult hepatitis B (OBI) infection among blood donors is of utmost importance [26]. The current method of detecting HBV infection by the Chemi Luminescent Immunoassay (CLIA) for the HBsAg marker is insufficient for diagnosing Occult HBV Infection (OBI). Consequently, the danger of transmitting OBI through blood transfusion remains significant [27]. The objective of this investigation was to examine the prevalence of Occult Blood Infections (OBI) among blood donors in the “Aseer” region for the duration spanning from January 2022 to December 2022. A total of 28,232 blood donor samples were obtained throughout the specified period, originating from 18 institutions located in the “Aseer” region, as explicitly identified. The variation in the number of donors among various hospitals can be attributed to the dependence of donor’s counts on the total population residing within the catchment region of each hospital. As an illustration, the proportion of blood donor samples obtained from ACH was 25%, whereas just 2% were obtained from PFKCC.
Out of the entire pool of blood donor samples, the proportion of samples testing positive for HBsAg was 0.47%, corresponding to a total of 133 positive samples. Based on the data provided, it was determined that a total of 85 individuals, accounting for 0.30% of the sample, tested positive for the presence of HBsAg. The samples serve as our focal point, and we compare these findings with the HBV DNA results obtained from Nucleic Acid Testing (NAT), as well as the outcomes pertaining to Anti-HBc antibodies. It was determined that only 16 out of the total blood donors, accounting for 18% of the sample, met the criteria for infection with OBI. Additionally, we have classified the two distinct categories of OBI and determined that 37.50% of the OBI population exhibited seropositivity, indicating the presence of anti-HBc antibodies. However, the majority of OBI positive donors, specifically 62.50%, had a non-reactive status for Anti-HBc, hence indicating seronegativity.
Based on our research findings, the prevalence of Occult Blood Infections (OBI) among blood donors in the “Aseer” region did not exhibit a statistically significant difference when compared to the prevalence observed in lower-resource countries, where the prevalence was found to be highly significant [28]. Several criteria are crucial in studying the incidence of occult Hepatitis B Infection (OBI), including the endemicity of Hepatitis B Virus (HBV), the diagnostic techniques employed, and various other factors [29]. The utilization of highly sensitive techniques, such as Hepatitis B Virus Nucleic Acid Testing (HBV NAT), is crucial in enhancing the identification and assessment of Occult Hepatitis B Infection (OBI) within the pool of blood donors [30].
One potential limitation of this study pertains to the duration of the study, since it would have been beneficial to include a time frame spanning more than one year in order to provide a more comprehensive analysis. The retrospective study holds significance in providing a comprehensive overview of the prevalence of Occult Hepatitis B Infection (OBI) among blood donors in the “Aseer” region. Consequently, a strategic approach can be devised to address and mitigate the transmission of OBI cases, thereby diminishing the occurrence within the region.
On the other hand, the transmission of OBI infection through blood transfusion is contingent upon the immune status of the recipients. Furthermore, genetic diversity is an additional element influencing the transmission of Occult Hepatitis B Infection (OBI) to recipients. Previous research has shown that there were significant genetic variations in HBV genotypes among donors from Southeast Asia [31]. Nevertheless, the occurrence of false negative findings remains a possibility even when employing NAT screening tests, as they may fail to identify low levels of viremia [32]. In order to mitigate this issue, certain blood services have implemented a pathogen inactivation procedure that involves the utilization of chemical agents to effectively eliminate the viral Deoxy Ribonucleic Acid/Ribonucleic Acid (DNA/ RNA) present in blood products. Furthermore, the administration of HBV vaccination to individuals serves as an additional preventive measure to mitigate the risk of acquiring OBI infection [33].
Conclusion
In summary, this study revealed the occurrence of OBI cases within the blood donor population in the “Aseer” region over the year 2022. The identification of these cases was achieved by utilizing serological markers of Occult Hepatitis B infection (OBI) on blood donor samples collected from 18 hospitals located in the “Aseer” region and received at central blood banks. The markers included serum tests for Hepatitis B surface Antigen (HBsAg) and antibodies against Hepatitis B core antigen (anti-HBc), as well as plasma tests for Hepatitis B virus (HBV) DNA. Our focus was on diagnosing Occult Hepatitis B Infection (OBI) cases by examining the presence of non-reactive HBsAg and positive HBV DNA, with or without the presence of anti-HBc reactivity. It is imperative to do additional research in order to ascertain the incidence of occult Hepatitis B Infection (OBI) in earlier years within the “Aseer” region. This will serve to provide a comprehensive foundation and database pertaining to these occurrences. Furthermore, it is strongly advised to establish a comprehensive policy outlining the appropriate procedures for managing and addressing Occupational Bloodborne Incident (OBI) cases. This policy should be designed to effectively mitigate the risk of transmitting hepatitis B virus infection to recipients of blood transfusions.
Declarations
Author contribution
A.K. concepted, designed the study and wrote the manuscript; A.M.A. designed the study and revised the manuscript; A.M.A, A.S.A, M.S.A and G.M.A. performed the research and collect the data; F.S.A. concepted, designed the study, analysed the data, supervised the research and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Deanship of Scientific Research at King Khalid University, Abha, Saudi Arabia through a project number (RGP2/426/44).
Institutional Review Board (IRB) statement
The study was conducted and approved by Asser Institutional Review Board (IRB) under number REC-04-09-2022 and date 09/12/2022.
Acknowledgments
We acknowledge the directorate of “Aseer” health affairs and “Aseer” regional laboratory for facilitate this study. Also, King Faisal Medical City for Southern Region for their support and King Khalid University for their funding under project number (RGP2/426/44).
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet. 2015; 386(10003): 1546-1555.
[Crossref] [Google Scholar] [PubMed]
- Zuckerman JN. Review: Hepatitis B immune globulin for prevention of hepatitis B infection. J Med Virol. 2007; 79(7):919-921.
[Crossref] [Google Scholar] [PubMed]
- Schmit N, Nayagam S, Thursz MR, Hallett TB. The global burden of chronic hepatitis B virus infection: Comparison of country-level prevalence estimates from four research groups. Int J Epidemiol. 2021; 50(2): 560-569.
[Crossref] [Google Scholar] [PubMed]
- Hagiwara S, Nishida N, Ida H, Ueshima K, Minami Y, Takita M, et al. Clinical implication of immune checkpoint inhibitor on the chronic hepatitis B virus infection. Hepatol Res. 2022; 52(9): 754-761.
[Crossref] [Google Scholar] [PubMed]
- Honkoop P, de Man RA, Niesters HG, Zondervan PE, Schalm SW. Acute exacerbation of chronic hepatitis B virus infection after withdrawal of lamivudine therapy. Hepatology. 2000; 32(3):635-639.
[Crossref] [Google Scholar] [PubMed]
- de Almeida Ponde RA. Detection of the serological markers hepatitis B virus surface antigen (HBsAg) and hepatitis B core IgM antibody (anti-HBcIgM) in the diagnosis of acute hepatitis B virus infection after recent exposure. Microbiol Immunol. 2022; 66(1): 1-9.
[Crossref] [Google Scholar] [PubMed]
- Kao JH. Diagnosis of hepatitis B virus infection through serological and virological markers. Expert Rev Gastroenterol Hepatol. 2008; 2(4): 553-562.
[Crossref] [Google Scholar] [PubMed]
- Carella A. The donation of blood for transfusion purposes. Considerations on 4 cases of death during collection of blood. Zacchia. 1967; 3(3):338-355.
- Sobel A. Blood transfusion transmission of viral diseases. Eur J Med. 1992; 1(2):67-68.
[Crossref] [Google Scholar] [PubMed]
- Seidl S, Kuhnl P. Transmission of diseases by blood transfusion. World J Surg. 1987;11(1): 30-35.
[Crossref] [Google Scholar] [PubMed]
- Oei W, Janssen MP, van der Poel CL. Modeling the transmission risk of emerging infectious diseases through blood transfusion. Transfusion. 2013; 53(7):1421-1428.
[Crossref] [Google Scholar] [PubMed]
- Keeffe EB. Hepatitis A and B superimposed on chronic liver disease: Vaccine-preventable diseases. Trans Am Clin Climatol Assoc. 2006; 117: 227-238.
[Crossref] [Google Scholar] [PubMed]
- Adekanle O, Ndububa DA, Ayodeji OO, Paul-Odo B, Folorunso TA. Sexual transmission of the hepatitis B virus among blood donors in a tertiary hospital in Nigeria. Singapore Med J. 2010; 51(12):944-947.
[Crossref] [Google Scholar] [PubMed]
- Zhang WZ, Li CQ, Ji GQ. Analysis on the effects of hepatitis B vaccine to prevent mother-to-children transmission of hepatitis B virus in Shunyi District of Beijing Municipal. Zhongguo yi Miao he Mian yi. 2010;16(2):136-139.
- Zou HB, Chen Y, Zhang H, Duan ZP, Jie LI, Zhuang H, et al. Prevention of hepatitis B virus vertical transmission: Current situation and challenges. Zhonghua Gan Zang Bing Za Zhi. 2010;18(7):556-558.
- Wu T, Kwok RM, Tran TT. Isolated anti-HBc: The relevance of hepatitis B core antibody-A review of new issues. Am J Gastroenterol. 2017; 112(12):1780-1788.
[Crossref] [Google Scholar] [PubMed]
- O'Flaherty N, Ushiro‐Lumb I, Pomeroy L, Ijaz S, Boland F, De Gascun C, et al. Transfusion-transmitted Hepatitis B Virus (HBV) infection from an Individual-Donation Nucleic Acid (ID-NAT) non-reactive donor. Vox Sang. 2018; 113(3):300-303.
[Crossref] [Google Scholar] [PubMed]
- Chen S, Ren F, Huang X, Xu L, Gao Y, Zhang X, et al. Underestimated prevalence of HIV, Hepatitis B Virus (HBV), and Hepatitis D Virus (HDV) triple infection globally: Systematic review and meta-analysis. JMIR Public Health Surveill. 2022; 8(11):37016.
[Crossref] [Google Scholar] [PubMed]
- Al-Raddadi RM, Dashash NA, Alghamdi HA, Alzahrani HS, Alsahafi AJ, Algarni AM, et al. Prevalence and predictors of hepatitis B in Jeddah City, Saudi Arabia: A population-based seroprevalence study. J Infect Dev Ctries. 2016; 10(10): 1116-1123.
[Crossref] [Google Scholar] [PubMed]
- Alzahrani FM, Shaikh SS, Alomar AI, Acharya S, Elhadi N. Prevalence of Hepatitis B Virus (HBV) among blood donors in Eastern Saudi Arabia: Results from a five-year retrospective study of HBV seromarkers. Ann Lab Med. 2019; 39(1):81-85.
[Crossref] [Google Scholar] [PubMed]
- AlRashdan Y, Al-Jaff K, Najdawi M. Occult hepatitis B in blood donation centers. J Med Life. 2023; 16(4):571-578.
[Crossref] [Google Scholar] [PubMed]
- He P, Zhang P, Fang Y, Han N, Yang W, Xia Z, et al. The role of HBV cccDNA in occult hepatitis B virus infection. Mol Cell Biochem. 2023; 478(10): 2297-2307.
[Crossref] [Google Scholar] [PubMed]
- Alter HJ, Tabor E, Meryman HT, Hoofnagle JH, Kahn RA, Holland PV, et al. Transmission of hepatitis B virus infection by transfusion of frozen-deglycerolized red blood cells. N Engl J Med. 1978; 298(12):637-642.
[Crossref] [Google Scholar] [PubMed]
- Korelitz JJ, Busch MP, Kleinman SH, Williams AE, Gilcher RO, Ownby HE, et al. A method for estimating hepatitis B virus incidence rates in volunteer blood donors. National heart, lung, and blood institute retrovirus epidemiology donor study. Transfusion. 1997; 37(6):634-640.
[Crossref] [Google Scholar] [PubMed]
- Dhingra N. Blood safety in the developing world and WHO initiatives. Vox Sang. 2002; 83(1):173-177.
[Crossref] [Google Scholar] [PubMed]
- Arababadi MK, Hassanshahi G, Pourfathollah AA, Zarandi ER, Kennedy D. Post-transfusion occult hepatitis B (OBI): A global challenge for blood recipients and health authorities. Hepat Mon. 2011; 11(9):714-718.
[Crossref] [Google Scholar] [PubMed]
- Kim YS. Definition, diagnosis, and prevalence of occult hepatitis B virus infection. Korean J Gastroenterol. 2013; 62(3):143-147.
- Mahmoud OA, Ghazal AA, Metwally DE, Elnour AM, Yousif GE. Detection of occult hepatitis B virus infection among blood donors in Sudan. J Egypt Public Health Assoc. 2013; 88(1):14-18.
[Crossref] [Google Scholar] [PubMed]
- Jayaraman S, Chalabi Z, Perel P, Guerriero C, Roberts I. The risk of transfusion-transmitted infections in sub-Saharan Africa. Transfusion. 2010; 50(2):433-442.
[Crossref] [Google Scholar] [PubMed]
- Lelie N, Busch M, Kleinman S. Residual risk of Transfusion-Transmitted Hepatitis B Virus (TT-HBV) infection by NAT-screened blood components: A review of observed versus modelled infectivity from donors with window period and occult HBV infections. Transfusion. 2021; 61(11):3190-3201.
[Crossref] [Google Scholar] [PubMed]
- Candotti D, Lin CK, Belkhiri D, Sakuldamrongpanich T, Biswas S, Lin S, et al. Occult hepatitis B infection in blood donors from South East Asia: Molecular characterisation and potential mechanisms of occurrence. Gut. 2012; 61(12):1744-1753.
[Crossref] [Google Scholar] [PubMed]
- Schlenke P. Pathogen inactivation technologies for cellular blood components: An update. Transfus Med Hemother. 2014. 41(4):309-325.
[Crossref] [Google Scholar] [PubMed]
- Seo DH, Whang DH, Song EY, Han KS. Occult hepatitis B virus infection and blood transfusion. World J Hepatol. 2015. 7(3):600-606.
[Crossref] [Google Scholar] [PubMed]
Citation: Khanum A, Alamri AM, Alshehr AM, Alsabrah AS, Alshehri MS, Albahja GM, et al (2024) Identification of Occult Hepatitis B Infection (OBI) among Blood Donors at “Aseer” Southern Region in Saudi Arabia. J Blood Disord Transfus. 15:572.
Copyright: © 2023 Khanum A et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

