Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Short Communication - (2023) Volume 14, Issue 5
High Consistency between Regulatory Decisions for New Drugs between the Swiss Swissmedic, the European Medicines Agency and the United States Food and Drug Administration
Ulrich P Rohr*, Anita Wolfer and Christine HaenggeliReceived: 15-Aug-2023, Manuscript No. JCRB-23- 22639 ; Editor assigned: 18-Aug-2023, Pre QC No. JCRB-23- 22639 (PQ); Reviewed: 31-Aug-2023, QC No. JCRB-23- 22639 ; Revised: 07-Sep-2023, Manuscript No. JCRB-23- 22639 (R); Published: 14-Sep-2023, DOI: 10.35248/2155-9627.23.14.470
Description
Drug Regulatory Agencies (DRAs) such as Swissmedic are often questioned on their interactions and differences in approval decisions [1]. The perception that there is a significant degree of divergent decision making and restriction in the label indication amongst the DRAs despite the same set of submitted clinical data packages lead us to this investigation. We analyzed the Swissmedic (SMC) regulatory decisions on New Active Substance (NAS) drug approvals with the United States Food and Drug Administration (FDA) and European Medicines Agency (EMA) decisions, focusing our analysis on the same drugs and data sets with particular focus on the oncology products over a 10-year-period between Jan 1st 2009 and Dec 31st, 2018. We compared approval rates, consensus decisions as well as divergent decisions on drug approvals between these three major agencies [2]. We particularly focused on divergent decisions between the three agencies and the underlying reasons. For the analyzed 10-year time period we identified 293 finalized regulatory decisions for NAS drug approvals for the three DRAs, 69 Oncology Products (OP) (including hematologic neoplasia) and 224 Non-Oncology Products (NOP) from all other therapeutic areas. For OP, approval rates at SMC were 88.4%, at EMA 91.3% and were highest at FDA with 95.7%. Using pairwise comparisons, these differences were however not statistically significant.
For NOP, approval rates at SMC, EMA and FDA were 86.2%, 93.8% and 88.8%, respectively. In pairwise comparison, only the difference between SMC and EMA reached statistical significance. This leads to a high consensus decision rate-either positive or negative decision- of 88.4% for OP and 84.4% for NOP indicating a substantial agreement in the decision making between the three DRAs. For the consensus approved OP, the indication wordings between DRAs were compared and no statistically significant difference or a specific pattern was observed among the three DRAs. In conclusion, no DRA was more restrictive in its decision in comparison to the 2 others. The outcome of our analysis indicates that the decision making between the analyzed DRAs is highly consistent. Consequently, the perception that one DRA is more or less restrictive than another is invalid. As a matter of fact, the divergent decisions across three major DRAs were low in number for OP (8/69=11.6%) as well as for NOP (35/224=15.6%).
In order to better understand the reasons of the 8 divergent decisions, we performed a subsequent research based on the assessment reports of the three agencies. The decision pattern of the eight OP is presented in Figure 1. SMC had the lowest rate of approval for those 8 OP (25%) followed by the EMA (50%) and the FDA with the highest rate (88%). The main reason for rejection by at least one DRA was the clinical trial design with uncontrolled phase II clinical data of the pivotal trial in 5 of 8 cases. Furthermore, in the remaining 3 cases, the patient number in the clinical studies was either low due to slow recruitment leading to premature closure or due to a different evaluation of the clinical meaningfulness of the trial outcome. To summarize, all 8 applications with divergent decisions came with major uncertainties which were recognized, evaluated and interpreted differently by the DRAs. This observation is in line with other reports (Figure 1) [3,4].
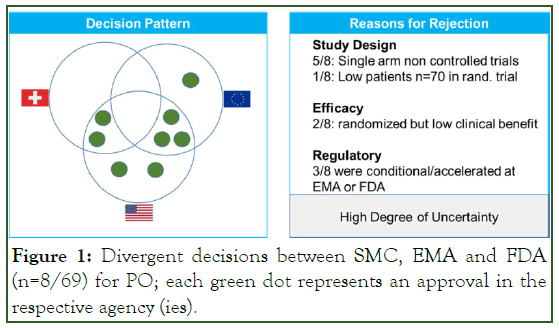
Figure 1: Divergent decisions between SMC, EMA and FDA (n=8/69) for PO; each green dot represents an approval in the respective agency (ies).
These clinical uncertainties also led in part to different approval pathways. For three applications, the FDA or the EMA granted an accelerated approval pathway or conditional marketing authorization pathway, respectively, in contrast to a regular drug approval. These two regulatory pathways for drug approval are usually limited to a certain duration and/or bound to the postmarketing obligation to provide additional clinical data for a conversion into a regular approval. As this type of regulatory pathway was only introduced at SMC in 2019 as a “temporary authorization pathway” all SMC regulatory decision before 2019 were either regular approvals or rejections. This most likely explains the lowest approval rate of 25% by SMC for these applications with a certain degree of uncertainty at the time of decision based on the benefit-risk assessment of the application.
In summary, we were able to show that for the investigated 10-year time period the decision making between SMC, EMA and FDA was highly consistent and independent of therapeutic area with a consensus decision rate of 84% for NOP and 88% for OP. For the few cases of divergent decisions for OP, the identified reasons were mainly a lack of robust randomized controlled trial design and interpretation of clinical benefit leading to high uncertainties for the benefit-risk assessment of the drug. Based on our 10-year period analysis on NAS we conclude that there is a high and significant alignment in the decisions among agencies and that there is no evidence on the perception that a particular agency stood out to be more restrictive in terms of approvals or indication restrictions as also reported by others [5,6]. This outcome is reassuring to us and further confirms that the 3 DRAs have a similar and consistent decision making amongst each other.
References
- Teixeira T, Kweder SL, Saint-Raymond A. Are the European Medicines Agency, US Food and Drug Administration, and other international regulators talking to each other? Clin Pharmacol Ther. 2020; 107(3):507-513.
- Rohr UP, Iovino M, Rudofsky L, Li Q, Juritz S, Gircys A et al. A decade comparison of regulatory decision patterns for oncology products to all other non-oncology products among Swissmedic, European Medicines Agency, and US Food and Drug Administration. Clin Transl Sci. 2023.
- Kashoki M, Hanaizi Z, Yordanova S, Vesely R, Bouygues C. A comparison of EMA and FDA decisions for new drug marketing applications 2014–2016: concordance, discordance, and why. Clin Pharmacol Ther. 2020; 107(1):195-202.
- Salcher-Konrad M, Naci H, Davis C. Approval of cancer drugs with uncertain therapeutic value: a comparison of regulatory decisions in Europe and the United States. The Milbank Q. 2020; 98(4): 1219-1256.
- Dalla Torre Di Sanguinetto S, Heinonen E, Antonov J, Bolte C. A comparative review of marketing authorization decisions in Switzerland, the EU, and the USA. Ther Innov Regul Sci. 2019; 53(1):86-94.
- Dorr P, Wadworth A, Wang T, McAuslane N, Liberti L. An analysis of regulatory timing and outcomes for new drug applications submitted to Swissmedic: comparison with the US food and drug administration and the European medicines Agency. Ther Innov Regul Sci. 2016;50(6):734-742.
Citation: Rohr UP, Wolfer A, Haenggeli C (2023) High Consistency between Regulatory Decisions for New Drugs between the Swiss Swissmedic, the European Medicines Agency and the United States Food and Drug Administration. J Clin Res Bioeth. 14:470.
Copyright: © 2023 Rohr UP, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

