Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 0, Issue 0
GM-CSF: Anti-Cancer Immune Response and Therapeutic Application
Susan Morand1, Monika Devanaboyina1, Courtney Fung1, Rachel Royfman1, Louis Filipiak1, Laura Stanbery2, Danae Hamouda1 and John Nemunaitis2*2Department of Medicine, The University of Toledo College of Medicine and Life Sciences, Carrollton, Gradalis, Inc., TX 75006, United States
Received: 08-Dec-2020 Published: 30-Dec-2020, DOI: 10.35248/2157-7560.21.S10.002
Abstract
A primary focus of cancer therapeutics today is precision, or target directed, therapy. Combination treatment with precision therapy can involve both immune and signal pathway targets. One approach involving the chemokine GMCSF involves enhancement of the immune system. Herein is a review of the literature and the current therapeutic role of GM-CSF, including the proposed immune mechanisms and potential applications of GM-CSF to enhance anticancer immunotherapy. GM-CSF’s potent effects on dendritic cell activation and subsequent stimulation of T-lymphocyte activity make it an attractive potential addition to combination therapeutic regimens, including radiation therapy, oncolytic viral therapy, immune checkpoint inhibition, and autologous tumor vaccines, and warrants further clinical exploration, with an emphasis on identifying concomitant molecular pathways that mediate resistance and sensitivity to the GM-CSF effect.
Keywords
Immunotherapy; Vaccines; Targeted therapeutics; Cancer; Immune cells
Introduction
Cancer therapeutics have rapidly expanded and presently include viruses, plasmids, and targeted cell therapeutics. More recently, cancer biologists have also turned to agents that target relevant molecular signals and immune response pathways which are enabling strategic combination opportunities. While the immune response to neoplasm is a physiologic process occurring daily, escape mechanisms can develop allowing for cancer proliferation.
To combat these mechanisms, several immune therapies have recently been developed and FDA approved as indicated therapy; these include several checkpoint inhibitors, which prevent blockade of the immune regulatory cells; Chimeric Antigen Receptor T-cell (CAR-T) therapies, which increase antigen targeting T cells; cell therapeutics activated by granulocyte-monocyte colony stimulating factor (GM-SCF); and prostatic acid phosphatase (PAP) and viral oncolytic therapeutics enhanced by GM-CSF expressing plasmid [1- 6]. Uses of immune stimulatory cytokines are an attractive direction for continued and expanded development in tumor research [7].
One cytokine established in current therapeutic use is Granulocyte- Monocyte Colony Stimulating Factor (GM-CSF). GM-CSF has primarily been studied as a hematopoietic growth factor, particularly enhancing the proliferation and differentiation of the precursors cells within the myeloid lineage [8]. In fact, this function has been utilized for years to enhance patient recovery following depletion of bone marrow cells, which is performed in preparation for a bone marrow transplant [9]. Recombinant human (RH) GM-CSF (sargramostim) was registered as indicated therapy by FDA for neutrophil recovery in patients with NLL, ALL, Hodgkin and non-Hodgkin lymphoma in March of 1991 [10]. In addition, GM-CSF use was also FDA approved as a component of sipuleucel-T. Sipuleucel-T is a therapeutic cancer vaccine that administers autologous Peripheral-Blood Mononuclear Cells (PBMCs) including Antigen Presenting Cells (APCs) that have been activated ex vivo with GM-CSF fused to prostate antigen and Prostatic Acid Phosphate (PAP). In the case of sipuleucel-T, the activated PBMCs have the capability to induce immune responses against PAP and inhibit cell proliferation signals with the aid of GM-CSF [11-15].
Recent data has revealed that GM-CSF not only acts as a hematopoietic growth factor, but also performs a significant role in immune modulation. One such mechanism includes assisting the immune system with recognizing cancer cell neoantigens, thereby mounting an effective immune response. In this role, not only does GM-CSF engage in proliferation and stimulation of the myeloid lineage [16-18], but also in the recruitment and activation of dendritic cells [19], the expression of Major Histocompatibility Complexes (MHC), the activation of T-cells [16,17] and the facilitation of immune response to cancer neoantigens [20]. As a result, GM-CSF can be an attractive addition to cancer therapeutic development.
Methods
GM-CSF molecular pathway
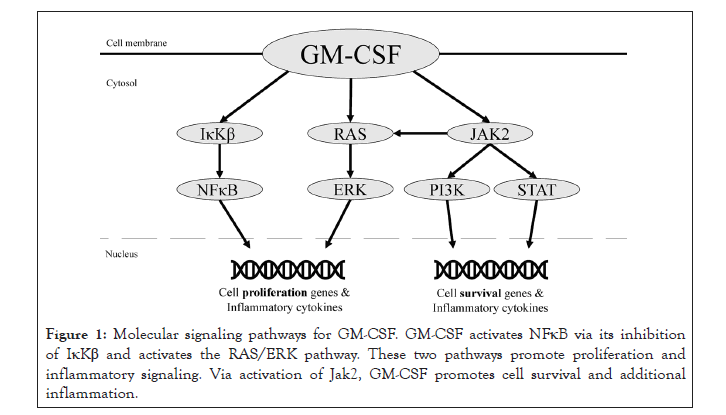
GM-CSF activates several pathways responsible for proliferation of hematopoietic cells and modulation of the immune system, including neutrophil and monocyte activation (Figure 1).
Figure 1: Molecular signaling pathways for GM-CSF. GM-CSF activates NFκB via its inhibition of IκKβ and activates the RAS/ERK pathway. These two pathways promote proliferation and inflammatory signaling. Via activation of Jak2, GM-CSF promotes cell survival and additional inflammation.
Functional activity is initiated by GM-CSF binding to an alpha subunit receptor (GM-CSFR α), responsible for signal activation, and beta subunit (GM-CSFRβ), responsible for signal transduction. After binding, protein synthesis and tyrosine phosphorylation cascades are engaged [21,22]. Affected pathways include NFκB, Jak2/Stat5, PI3K-Akt, and ERK1/2, all of which contribute to cell differentiation via transcription of gene products [16-18]. GM-CSF has been shown to activate nuclear factor kappa-lightchain- enhancer of activated B cells (NFκB), a protein complex that contributes to the inflammatory response [23]. Ordinarily, NFκB is bound by the IκB kinase complex in the inactivate state. When IκB is phosphorylated, it releases NFκB and is degraded in the proteasome. Subsequently, free NFκB may translocate to the nucleus to stimulate transcription of immune and inflammatory cytokines [24]. GM-CSF’s interaction with GM-CSFR is required for this release. The α- and β-chains of GM-CSFR interact with IκB kinase beta (IκKβ), which is one of three components that make up the IκB kinase complex. In the presence of GM-CSF, the GM-CSFR α chain interacts with IκKβ, resulting in the release of NFκB [25]. Once NFκB is activated, multiple sequential downstream effects occur, including the activation, proliferation, and differentiation of T- and B-cells [26].
Beyond its role with NFκB, GM-CSF also initiates the Janus Kinase (JAK) and Signal Transduce and Activation of Transcription (STAT) pathway [22]. STAT proteins then translocate to the nucleus to modify gene transcription, including Bcl2, an important gene in regulating apoptosis [27]. Physiologically, the JAK/STAT pathway is involved in multiple signaling mechanisms responsible for activating cytokines, growth factors, cell proliferation, and differentiation. These events are essential for hematopoiesis and immune development [28]. In addition, JAK/STAT is involved in MHC expression [29]. IFN-γ is important in inducing MHC II molecules in nucleated cells through JAK/STAT signaling which further contribute to the immune response [30].
Finally, JAK2 phosphorylation also activates PI3K and ERK1/2 along with the MEK/ERK pathway [31]. These two pathways work in concert with the aforementioned pathways. Cell survival is dependent on PI3K and JAK/STAT5-Bcl2 signaling, while cell proliferation occurs with NFκB and MEK/ERK1/2 signaling. In conclusion, GM-CSF controls differentiation and survival of key immune effector cells, including macrophages, granulocytes and eosinophils through involvement and interaction with NFκB and JAK2 [16-18].
GM-CSF in cancer
GM-CSF is commonly found within the tumor microenvironment [32,33]. Expression of GM-CSF has been linked to both tumor proliferation and tumor inhibitory activity [16-18,33]. The effects of GM-CSF (whether pro- or anti-tumor) may be dependent upon the type of tumor. In colorectal cancer, an elevation of serum GMCSF correlated with a better prognostic outcome. In fact, colorectal cancer cells that produced GM-CSF were more often diagnosed at a lower tumor stage, and these patients had a prolonged survival rate compared to those cells which did not produce GM-CSF [34]. In contrast, patients with glioblastoma multiforme experienced a poorer prognosis and worse tumor grade with increased levels of GM-CSF and GM-CSFR [35]. Similarly, elevated GM-CSF was also associated with a poorer prognosis in squamous cell carcinoma of the head and neck [18]. The mechanism to explain differential effects is not well understood but is likely dependent on proteins and other cytokines in the tumor microenvironment that interact with GM-CSF to mediate tumor genesis or inhibit tumor growth [20].
Effects on immune cells
Stimulation of myeloid lineage: GM-CSF is a major growth factor in myeloid stem cell differentiation to granulocyte and macrophage progenitor cells [36-40]. Lung epithelial cells, uterine cells, vascular endothelial cells, hematopoietic stem cells and fibroblasts also all express GM-CSFR α, which mediates additional response to GMCSF. Similarly, monocytes, macrophages, neutrophils, eosinophils, basophils, and dendritic cells express a cognate receptor for GMCSF. In contrast, lymphoid cells do not express GM-CSFR α, including T-cells, NK cells, and B-cells (CD19+). Consequently, GM-CSF’s direct effects are generally limited to the myeloid lineage rather than the lymphoid lineage under normal conditions [41]. However, studies have shown that hematopoietic malignancies may express GM-CSFR α, which represents a direct interaction between GM-CSF and lymphoid cells [16,41]. Other studies have also shown that malignancies, such as hairy-cell leukemia, breast cancer, lung cancer, and B cell malignancy, may express GM-CSFR α, which may α represent a direct interaction between GM-CSF and lymphoid cells in the tumor microenvironment [16,41,42].
Information regarding the peripheral mechanism of GM-CSF can be gleaned from studies of inflammatory conditions such as Rheumatoid Arthritis (RA). One study compared twice monthly administration of mavrilimumab, an anti-GM-CSFR α antibody, versus placebo in 305 patients with RA. Researchers then compared serum biomarkers and whole blood gene expression profiles between the groups. In patients receiving the antibody, there was decreased expression of myeloid cells and reduced T cell activation [43]. Mavrilimumab was also associated with a therapeutic response, underscoring GM-CSF’s proinflammatory activity [44]. Another study examined an injectable, DNA-encoded GM-CSF in order to understand its impact on monocyte derived Langerhans Cells (LCs). Following injection into the skin of mice, researchers found increased density of LCs at the inoculation site, indicating proliferation of monocytes in the presence of GM-CSF [45]. Thus, GM-CSF stimulates the myeloid lineage both within the bone marrow and more peripherally, as evidenced by the LC study.
Differentiation of Dendritic Cells (DCs) from monocytes: It is well established that GM-CSF promotes differentiation of monocytes into Dendritic Cells (DCs) under both inflammatory and steady-state conditions [17,46-48]. DCs express CD11c and MHC-II, whereas macrophages do not [49]. Although more recent data suggests that the two monocyte derivatives are more similar than previously thought (macrophages may also express MHC-II), DCs alone express CD11c, CD80, and CD86, which are important costimulatory molecules expressed by APCs to activate T-cells [50]. This indicates that GM-CSF can promote a class of phagocytic cells that can present antigens to T-cells, thereby enhancing an antitumor response [51].
The common dendritic Cell Precursor Cell (CDP) may differentiate into three major DC subsets: migratory, Lymphoid-Resident (LR), and plasmacytoid (pDC) [51,52]. Each subset differs in its response to GM-CSF. First, differentiation of migratory DCs requires GM-CSF. Conversely, LR DCs rely very little on GM-CSF for differentiation. The plasmacytoid subgroup’s response to GMCSF is dependent upon its stage of differentiation. It appears that the pDC lineage is inhibited by GM-CSF stimulation of the CDP; however, terminal differentiation of pDC precursors to pDCs is likely enhanced by DCs [17]. Clinical research supports these findings; a GM-CSF vaccine was shown to exhibit enhanced antitumor immunity through CD8α–, CD11c+ DC expression [53]. This subset of DCs are categorized as migratory dendritic cells and correlate with tumor cell phagocytosis and anti-tumor immunity through the expression of costimulatory molecules [21,54,55]. Therefore, GM-CSF promotes both the general DC lineage along with the most highly anti-tumor subset of DCs.
Recruitment of Dendritic Cells (DCs): While the mechanism by which GM-CSF recruits DCs is still under investigation, the current hypothesis proposes that GM-CSF causes the release of chemoattractant to recruit DCs, including C-C motif chemokine ligand 2 (CCL22). To this point, a study analyzed GM-CSF’s role in murine colon specimens that had been infected with C. rodentium
In the event of an infection, increased levels of chemokine CCL22 were noted along with DCs localized to the lamina propria of the GI mucosa. However, in GM-CSF-/- mice, there was no increase in DC infiltration, nor an increase in CCL22. Expectedly, the GM-CSF-/- mice had increased bacterial burden of C. rodentium Interestingly, administering GM-CSF to the GM-CSF-/- mice reversed the deficient DC response [19].
Notably, GM-CSF’s chemokine up regulation was specific to the chemokine CCL22; in contrast, chemokine CCL8 was expressed in similar levels by wild type and GM-CSF-/- mice infected with C. rodentium To further explore CCL22’s role in DC recruitment, wild type mice were injected with anti-CCL22 antibody or control IgG. After two weeks, the anti-CCL22 group showed a significant increase in bacterial colonization compared to the control IgG group, despite the fact that both had functional GM-CSF. These results highlight GM-CSF’s role in DC recruitment, which is likely mediated by chemokines including CCL22 [19].
Other studies highlight additional chemokines induced by GMCSF. Particularly, researchers administered anti-GM-CSF antibody to a murine Clostridium difficile model and found decreased levels of TNF-α, IL-1β, iNOS, CXCL1, and CXCL2, but no changes in CCL2, CCL4, CXCL9, or CXCL10. CCL22 was not analyzed in this study [56]. Another study examining GM-CSF in bone marrow found increased levels of CCL1, but not CCL5, upon administration of GM-CSF to murine bone marrow macrophages [57]. Dendritic cells express receptors complementary to several of these chemokine ligands, further emphasizing the importance of GM-CSF chemokine induction in DC migration [19,58].
Beyond attracting DCs to the site of an infection, there is evidence that GM-CSF recruits DCs to draining lymph nodes [21]. In a study of anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA4) cancer antibodies, co-administration of GM-CSF increased DC density in the draining lymph node, thereby increase the exposure of cancer antigens to T cells in the paracortex, as well as in the spleen, leading to an increased tumor-specific T-cell response [59]. Similarly, the aforementioned study involving DNA-encoded GMCSF injections into mice found increased recruitment of DCs to draining lymph nodes, as the cells more than doubled in GMCSF injected murine models when compared to models without treatment [45].
Finally, a study of tuberculosis observed the effects of differential expression of GM-CS in target organs. In their study, mice were engineered to overexpress GM-CSF in the lungs with knock-out of GM-CSF in other organs. They noted adequate recruitment of both T-cells and macrophages to the site of infection (lungs) along with production of IFN-γ and TNF-α, but impaired long-term granulomatous response at 60-90 days, resulting in murine demise. They attribute these findings to adequate production of certain chemokines such as RANTES alongside deficient expression of lymphotactin and MIP-1 β [60]. Therefore, GM-CSF’s role in chemokine induction may be dependent on distribution of GMCSF through target organs.
Activation of Dendritic Cells (DCs) and enhanced expression of Major Histocompatibility Complexes (MHC): The mechanism by which GM-CSF activates DCs can be elucidated through study of known GM-CSF-mediated inflammation and hostdefense pathways. Broadly, GM-CSF induces phagocytosis at the site of inflammation as a form of protection against pathogens [61,62]. However, the type of pathogen dictates the class of phagocyte utilized, which may include monocytes, macrophages, granulocytes, and DCs [49,63,64]. Of particular interest, DCs are monocyte-derived cells that can be programmed by GM-CSF [65]. Upon activation of DCs, GM-CSF promotes up regulation of genes for inflammasome function, chemotaxis, and phagocytosis. A potential outcome is induction of inflammatory cell death via pyroptosis, which is activated by the inflammasome [49,64]. Pyroptosis is Caspase 1-dependent programmed cell death, which is characterized by the induction of inflammatory cytokines including IL-1β and IL-18, along with rapid cell death by plasma membrane rupture and release of proinflammatory cytosolic material [66]. GM-CSF promotes this manner of cell death [49,64].
GM-CSF also plays an important role in the expression of MHC by DCs. A murine study found that GM-CSF alone or in combination with interleukin-4 (IL-4) both elicited an increase in MHC class II (MHC-II) expression on DCs [67]. A similar study corroborates the finding that GM-CSF alone leads to extensive MHC-II expression and proliferation of DCs in liver-derived murine cells [68]. Alternatively, GM-CSF can increase basophil expression of MHCII. Basophils normally do not express MHC-II, but when GM-CSF is combined with various cytokines (i.e. combination with IFNγ), MHC-II expression has been demonstrated in basophils [69].
Others have also demonstrated GM-CSF induced an increase in mRNA levels of class II Trans activator (CIITA), a crucial regulator of MHC-II expression in human derived monocytes. GM-CSF specifically increased expression of CIITA types I and III, which resulted in an increase of both total protein and RNA of MHCII molecules. IFN-γ, however, increased CIITA types III and IV. Since GM-CSF and IFN-γ both increased CIITA type III, this molecule may be amplified in co-expression of GM-CSF and IFN-γ, perhaps explaining the significant increase in MHC-II expression in the study by Voskamp et al. with combination GM-CSF and IFNγ compared to when GM-CSF and IFN-γ were administered separately [69,70].
In contrast to the evidence linking GM-CSF to MHC-II expression, there is limited data to demonstrate GM-CSF’s effect on expression of MHC class I (MHC class II) molecules. In a pre-clinical study, GM-CSF increased the levels of MHC-I molecules and boosted the anti-tumor immune response, though the study does not describe the mechanism behind this effect [71]. In another study investigating GM-CSF’s relationship with MHC-I, it is suggested that GM-CSF expression results in low levels of the class I molecules by regulating the invariant chain (Ii) in myelomonocytic cells in the absence of MHC-II molecules. The Ii has a strong association with class II molecules through its interaction with the class II peptide-binding groove. It is inferred that this same Ii portion can bind to the class I peptide binding groove. However, Ii has a much greater affinity for MHC-II than MHC-I; therefore, in normal conditions GM-CSF yields low levels of MHC-I relative to MHC-II [72]. The relationship between GM-CSF and MHC is further described by clinical trials employing GM-CSF and subsequently noting increases in MHC-I and/or MHC-II. Some of these therapeutics are discussed later in this review.
Effects on T-cells: While GM-CSF is frequently secreted by T-cells to activate neutrophils, macrophages, eosinophils, and basophils [73], GM-CSF has been shown to indirectly cause proliferation of CD4+ and CD8+ T-lymphocytes [74]. Following expansion of macrophage and dendritic cell lineages with GM-CSF, these immune cells subsequently serve as Antigen Presenting Cells (APC) supporting an antigen-induced immune response capacity [67]. Further, GMCSF increases the frequency of CD4+ and CD25+ T cells (the regulatory-cell subset), which is correlated to high density of MHCII and B7 (CD80) on DCs [21,67]. Although this study suggests promotion of a T regulatory subtype, it also highlights enhanced expression of costimulatory molecules and MHC, which feeds back to enhance T-cell function.
Furthermore, GM-CSF modulates differentiation of helper T cell subtypes: T-cell helper subtype 1 and 2 (Th1 and Th2). Mice lacking GM-CSF died upon exposure to Mycobacterium tuberculosis due to the inability to produce a Th1 response [21,60]. In another study, an HIV-1 vaccine containing gp120 and GM-CSF elicited a seven-fold increase in IFN-γ, implying a greater Th1 response [75]. Although, it was also shown that the GM-CSF Th1-specific response enhanced by IFN-γ subsequently exacerbated autoimmune disorders such as multiple sclerosis [73,76]. G250-GM-CSF fusion gene is an experimental cancer therapeutic that has been tested and also demonstrated significant Th1 and Th2 response related to the GM-CSF component in support of anti-cancer activity. G250 is a widely expressed renal cell associated antigen and immune response with elevation in CD3 and CD4 cell populates along with activation of immunomodulating dendritic cells against renal cell cancer was demonstrated [77].
The aforementioned study involving DNA-encoded GMCSF injections into mice found improved activation of T-cells versus poorly immunogenic tumor antigens, including peptide immunization of skin sites with mutant p53. In contrast, control cells did not mount any detectable T-cell response versus this peptide. Finally, this study also found more rapid and robust expression of antibodies in the GM-CSF injected mice following immunization with a common melanoma DNA segment encoding a tyrosinase, indicating a potential role for B-lymphocytes [45].
GM-CSF anticancer clinical applications and trials
Several novel therapeutics are in development to integrate GMCSF in the treatment of cancer. These therapeutics utilize a wide breadth of delivery vehicles, including plasmid DNA, oncolytic viruses expressing GM-CSF, and recombinant GM-CSF. The hope is that by integrating GM-CSF into treatment regimens, existing immune treatments can be enhanced to more effectively control cancer and to induce durable periods of remission. As detailed in this review, GM-CSF interacts with multiple immune modalities, including Dendritic Cells (DCs), helper T cells, and cytotoxic T cells. Through enhancement of their individual and collective functions, GM-CSF enhances the body’s anticancer immune activity, which makes it an attractive mechanism to target tumor cells.
Previous clinical studies have shown enhanced anti-tumor responses with the use of GM-CSF as a therapeutic. Systemic therapy with recombinant GM-CSF was associated with an increase in Prostate- Specific Antigen (PSA) specific CD4+ T cell and CD8+ T cell precursors among treated prostate cancer patients. In metastatic castration-resistant prostate cancer specifically, sipuleucel-T was developed as a therapeutic vaccine consisting of autologous Peripheral-Blood Mononuclear Cells (PBMCs) with activation by PA2024 recombinant fusion protein of a prostate antigen fused with GM-CSF [11-15]. In a multicenter phase III trial, 341 patients received sipuleucel-T and 171 received placebo treatment of cells expressing costimulatory CD54 molecule, and all patients enrolled in the study received previous combined androgen blockade therapy. The results show a significant improvement in overall survival where treatment group was 25.8 months and control group was 21.7 months. For 3-year survival, 31.7% for treatment group was compared with 23% for placebo. In addition, the survival improved across subgroups, such as increased PSA level [78]. This study further emphasizes the capability of GM-CSF to activate T cells and associated cytokines for cancer therapy, and sipuleucel-T was approved by the FDA in 2010 with the observed beneficial outcomes [15].
GM-CSF protein has also been tested as a therapeutic for advanced melanoma. A phase III trial administered adjuvant GM-CSF (sargramostim) peptide vaccine on days 1 through 14 on 28 days cycles for a total of 13 cycles of treatment or placebo peptide vaccine to completely resected stage IV or high-risk stage III melanoma patients after IFN-α-2b therapy. Median overall survival between treatment and placebo appeared improved (69.6 vs. 59.3 months, respectively), but was not statistically significant. However, there was no difference in adverse events between treatment and placebo, indicating GM-CSF treatment can be safely administered. Although insufficient evidence of anticancer activity related to GM-CSF protein was observed in one trial, it was concluded that a different subset of melanoma patients with resected visceral melanoma metastases could benefit from GM-CSF therapy. In addition, GM-CSF could be combined with other therapies to provide a statistically significant response [79].
GM-CSF in combination with radiation: GM-CSF can be considered as an experimental adjuvant to radiation therapy. Radiation therapy can yield a systemic response outside of the targeted area through the immune system (abscopal effect) [80]. Abscopal response refers to the phenomenon in which systemic chemotherapy is enhanced following local irradiation of a tumor. It is suspected to be due to release of tumor antigens from dying irradiated cells, which spur an improved immune response to distant cancer sites [81,82]. Promising results have been exhibited in a study observing abscopal responses for metastatic solid tumors. In one study, 11 out of 41 patients presented with a positive abscopal response after treatment with radiation and GM-CSF, the overall survival improved from 8.33 to 20.98 months (95% CI 14.2 to 42.9) [83]. Further clinical trials of radiation therapy supplemented with GM-CSF are ongoing [84].
GM-CSF Vaccine (GVAX): GVAX involves a tumor cell transfection of GMSCF plasmid to stimulate anticancer immune response [85,86]. This immunotherapy has shown promising results as a cancer therapeutic in pre-clinical and clinical studies involving various types of cancer [87-89].
Promising results of immune activation have been extensively demonstrated with GVAX in vitro and in vivo [85,86,90,91]. In one clinical study, GVAX was used for 20 patients with stage IIBIV melanoma, and increase in serum GM-CSF levels along with an increased immune response and decreased levels of myeloidderived suppressor cells was observed [92]. In another phase I study of glioblastoma patients, GVAX was administered to 10 patients and demonstrated enhanced immune responses with significantly increased expression of CTLA-4, PD-1, 4-1BB, and OX40 by CD4+ cells and PD-1 and 4-1BB by CD8+ T cells [93].
GVAX was also sequentially administered to prostate cancer patients for four treatment cycles as adjuvant therapy after docetaxel at an initial dose of 5 x 108 cells followed by 3 x 108 cells for three more doses followed by an additional 6 doses of GVAX post radical prostatectomy in 5 patients. The results showed a median drop of 1.47 ng/ml for PSA. All five patients completing treatment had undetectable PSA levels 3 years after radical prostatectomy. It is also important to note that no grade 3 or 4 adverse effects were noted in any of the patients [94]. Following these promising results, two phase III clinical trials were initiated in prostate cancer, VITAL-1 and VITAL-2. These trials were designed to investigate GVAX compared to standard of care chemotherapy. The VITAL-1 study randomized castration resistant prostate cancer patients to receive GVAX or docetaxel, was terminated early when analysis showed no therapeutic benefit. However, subset analysis showed that patients with a projected survival ≥18 months have a hazard ratio of 0.90 (95% CI:0.61-1.33) meaning that immunotherapy could improve outcomes compared to chemotherapy alone. In terms of safety concerns, this study reported grade 3, 4, or 5 adverse events at a rate of 25% and majority of the deaths were attributed to pancreatic cancer progression. Neutropenia was noted to be the most common grade 3-5 adverse event with 42 out of 278 (15%) in the chemotherapy group, and the total number of grade 3-5 adverse events were reported at a higher rate in chemotherapy at 16.9% than for immunotherapy at 4.2%. The most common grade 3-5 adverse event for immunotherapy was fatigue in 7 out of 307 (2%), meaning that adverse events could still occur with GM-CSF therapy, but death related to serious adverse events was unlikely due to the treatment of GM-CSF [95,96].
GVAX has also been tested in other solid tumors including NSCLC. In 43 patients with early stage or advanced NSCLC, 3 exhibited a complete response, with two remaining without disease after 5 years demonstrating a durable response [97]. A subsequent trial which enrolled 83 patients showed similar results, with 3 patients achieving a durable complete response [98]. While these results were promising, following the results of the VITAL-1 and -2 trials, no phase III trials were developed.
A bystander GVAX vaccine has also been explored to potentially expand GVAX activity. A phase I trial evaluating safety of the vaccine demonstrated common adverse local effects including erythema, swelling and pruritis in a majority of the patients, with limited grade 3 or 4 adverse events. The study did not show a partial or complete tumor response in the 49 treated patients, so combination studies with other agents were recommended [85]. Further research involving GM-CSF based vaccines is ongoing [99].
Oncolytic viruses employing GM-CSF: Another manner in which GM-CSF has been employed in anticancer immunity is through the use of oncolytic viruses [100]. The mechanism behind oncolytic viruses attacking cancer cells is two-fold. First, viruses infect cancer cells; this step has been particularly studied in melanoma, colorectal carcinoma, metastatic pancreatic carcinoma, and multiple myeloma. Cancer cells have defective IFN-γ response and are subsequently more likely to accept viral material in the intracellular compartment [100]. In this way, viruses such as the herpesvirus, adenovirus and vaccinia virus can enact lytic processes within tumor cells, slowing the growth of the overall cancer through cell death [101-103]. Second, as viruses are taken into these cells, the infection may create an “inflammatory storm” in the tumor microenvironment, attracting both an innate and adaptive immune response to the cancer cells [104]. Because cytotoxic CD8+ T-cells must lyse the host cell of a virus to clear the perceived threat, the cancer cell itself is destroyed immunologically [104]. Concurrent with the inflammatory storm, many cytokines are released in the tumor microenvironment to attract the immune response. GM-CSF in particular enhances the stimulation of neutrophils, dendritic cells, eosinophils, basophils and macrophages to enhance both the innate and adaptive immune response [104]. Therapeutically, this effect may be enhanced with viral gene manipulation, through which a gene encoding the expression of GM-CSF is incorporated into the viral genome prior to its injection into the tumor site [104]. The addition of GM-CSF to multiple oncolytic viral vehicles, such as the herpesvirus, adenovirus, and vaccinia virus have shown the ability to trigger a significant clinical antitumor immune response compared to non-transfected oncolytic viruses [101-104].
In one particular study, the anti-tumor effects of the herpes virus strain NV 1034, which expressed GM-CSF, were compared to NV 1023, which did not express GM-CSF [101]. The NV 1034 strain displayed a significantly greater antitumor reaction than the NV 1023 strain in mice. Importantly, these two strains did not perform significantly differently in mice that were depleted of CD4+ and CD8+ T-cells, underscoring the proposed mechanism that GMCSF enhances immune effector activity versus cancer [101].
A second study conjugated the GM-CSF gene with a cancer-specific E2F promoter region to create GM-E2F, which was incorporated into an adenoviral genome [102]. E2F is a transcription factor that regulates the progression from G1 to S of the cell cycle. Many cancers up regulate E2F, particularly tumors with mutant Retinoblastoma (Rb), which tightly regulates E2F to prevent aberrant progression through the cell cycle [105]. In oncolytic viruses, E2F promoter regions have been incorporated to increase specificity for cancer cells rather than healthy cells. Mechanistically, if the promoter is activated by a protein specific to the cancer, then the protein (in this case, GM-CSF) should be expressed more highly in malignant versus normal cells [106].
In the study employing GM-E2F, GM-CSF was found to be efficacious in producing antitumor effects, consistent with prior studies. Additionally, the GM-E2F oncolytic virus was more effective than the E2F oncolytic virus alone in mice, even if the mice were immunodeficient. Lastly, the tumors treated GM-E2F were found to have eosinophilic infiltrate into the tumor, while EF2-treated tumors were not, demonstrating that GM-CSF may actually alter the composition of the immune response rather than just enhancing it [102].
Finally, a third study incorporated GM-CSF into the Guang 9 (VG9) strain of vaccinia viruses [103]. This too produced a strong tumoricidal response in a mice melanoma model, with notable inhibition of tumor growth, prolonged-survival and a cytotoxic response. The immune response was measured via antibodies against the tumor, which continued to increase 21 days following injection with the GM-CSF strain; levels declined after 21 days in the non-GM-CSF strain [103].
Taken together, these studies evaluated various effects of GM-CSF when combined with a myriad of viruses. In sum, GM-CSF appears to play a vital role in the immunogenic response to oncolytic viruses and significantly enhance the treatment’s effects.
One major obstacle to the use of oncolytic viruses is induction of neutralizing antibody as early as 3-4 weeks after first dose thereby limiting prolonged anticancer activity. This is especially true if the vector is a common virus, in which case the patient’s body has likely developed immune memory [107]. Therefore, several steps must be taken initially in order to prevent the immune system from neutralizing the virus. The most straightforward of these initialsteps is to inject the oncolytic virus directly into the tumor, which is a common route of administration of oncolytic viruses [104,107]. However, this is not feasible in all metastatic or systemic cancers [100,104].
Another option, therefore, includes combining oncolytic virus treatment with chemotherapy. In this way, one can initially suppress the immune response via chemotherapeutic agents, which would allow the virus to infect vulnerable cancer cells and begin the lytic process. Once the immune system recovers from chemotherapy, it can then attack the infected cells [100,107]. The recovery of the immune system in this phase may be accelerated by incorporating cytokines, including GM-CSF.
A third option is injection of the virus into the patient with a protective coat [100,107]. The virus would then be protected extracellularly from the immune response, preventing neutralization. Examples of these coats include liposomes and a polyvalent diazonium polymer [100,107].
A final option is the “Trojan horse” mechanism, whereby immune cells (usually cytotoxic T-cells, but also natural killer cells, monocytes, dendritic cells or endothelial cells) are removed from the body and infected with the oncolytic virus [100,107]. These cells are then injected back into the patient and the virus is safely hidden within the host cells from an immune reaction [100,107]. When these cells respond to tumor antigens, the virus becomes free to infect cancer cells, express GM-CSF and spur an immune response to the cancer. In short, the antitumor effects of oncolytic viruses can be greatly enhanced by the addition of GM-CSF into the viral genome. At the same time, steps must be taken to ensure that these viruses reach cancer cells before being destroyed by the immune system too early.
Clinical trials employing GM-CSF as a cancer therapeutic: OncoVEXGM-CSF, also known as Talimogene Laherparepvec (T-VEC) is an oncolytic virus derived from human Herpes Simplex Virus 1 (HSV-1) with insertion of the GM-CSF gene. T-VEC was the first oncolytic virus therapy to receive FDA approval for significant clinical benefit and safe administration in advanced melanoma patients in 2015 [11]. In a phase II trial for metastatic melanoma patients, T-VEC was given to 50 patients who previously did not respond to standard therapy of dacarbazine/temozolomide or ILD- 2. Thirteen patients (26%) reported complete or partial response after a median follow up of 18 months. In addition, overall survival was 58% (T-VEC) versus 40% (control) for patients with stage IV disease at one year, which justified initiation of a phase III trial [4]. The OPTiM trial involved 436 patients, 295 received T-VEC and 141 received subcutaneous recombinant GM-CSF. Median overall survival was 23.3 months (95% CI:19.5-19.6) for T-VEC and 18.9 months (95% CI: 16.0-23.7) (HR: 0.79; 95% CI, 0.62- 1.00; p=0.0494) for control. The Objective Response Rate (ORR) consisting of complete and partial responses was 31.5% (95% CI:26.3–37.2) for T-VEC and 6.4% (95% CI: 3.0–11.8) for control. T-VEC significantly demonstrated improved responses over GMCSF alone (p<0.0001) [12].
Vigil; autologous tumor vaccine+rhGM-CSF cDNA and bishRNAfurin: Vigil is an autologous tumor cell vaccine constructed from fresh autologous tumor tissue and transfected ex vivo with a multigenic plasmid encoding a GM-CSF DNA expressive unit and a bifunctional short hairpin RNA (bi-shRNAfurin), whose mechanism is to suppress furin and the downstream expression of TGFβ1 and TGFβ2 [108]. Furin is a protease that cleaves TGFβ proprotein into its active TGFβ1 and TGFβ2 derivatives. By blocking the translation of furin, Vigil minimizes the downstream immunosuppressive effects of TGFβ, allowing immune cells to infiltrate the tumor microenvironment. Combination of GMCSF expression and knockdown of TGFβ1 and TGFβ2 mediate synergistic mechanisms to improve immune function versus cancer. Additionally, the autologous tumor vaccine introduces the personal tumor neoantigens to the immune repertoire thus priming T cells to the individual tumor neoantigens. Educating immune effector T cells towards cancer specific neoantigens will optimize the response specifically against the invading cancer and would be predicted to minimize off target toxicity related to immune response including Vigil and/or combination immunotherapies (i.e. checkpoint inhibitors) [109]. With these 3 mechanisms (neoantigen identification, GM-CSF expression, and TGFβ knockdown) working in concert, Vigil empowers the immune system to identify and eliminate cancer cells [109].
Phase I and II trials of Vigil have demonstrated a remarkable safety profile along with anti-tumor activity against several solid tumors, Ewing’s sarcoma and melanoma [110-116]. Vigil treated Ewing sarcoma patients (N=16) were compared with a contemporaneous group of Ewing sarcoma patients that did not receive Vigil (N=12) over a period of 3 years. During that period, the Vigil treated group received a monthly injection of Vigil, to which they experienced no ≥ Grade 3 toxicities. The Vigil treated group saw a 1-year survival of 73%, compared to only 23% in the non-Vigil group. The Vigil treated group also had a median overall survival of 731 days compared to 207 days in the control group [93].
A phase I trial of advanced stage solid tumor patients demonstrated safety of Vigil. In addition, γ-IFN-ELISPOT spot positive response was correlated with prolonged survival in these patients [110]. Long term follow up of 3 years continued to demonstrate improved overall survival correlation with γ-IFN-ELISPOT indicating that Vigil is able to activate an immune response [111]. Additionally, Vigil is able to increase levels of CD4+/CD8+ T cells in advanced cancer patients [117]. In ovarian cancer, a phase II trial demonstrated safety with no Grade 3/4 toxic events observed. In addition, γ-IFN-ELISPOT positivity was increased post treatment with Vigil and correlated with improved RFS [118,119]. A follow up Phase IIb randomized trial in ovarian cancer was recently completed. Significant clinical benefit in both RFS and OS was found in tumors with BRCA wild type expression [120]. This may be attributed to intact homologous recombination machinery and therefore more clonal versus sub clonal neoantigens [121,122]. Collectively these results suggest that Vigil is able to activate the immune system, specifically through the induction of memory T cells to induce durable tumor responses.
GM-CSF in combination with Immune Checkpoint Inhibitors (ICIs): One may also consider GM-CSF as an adjunct to Immune Checkpoint Inhibitor (ICI) therapy. Immune checkpoint inhibitors mitigate immunosuppressive mechanisms of cancer cells by blocking the interaction of PD-L1 with PD-1 or CTLA-4 with CD80/86. Physiologically, the interaction of PD-L1:PD-1 or CTLA-4:CD80/86 indicates that an immune cell has bound a selfcell. To prevent autoimmunity, these immune checkpoints inhibit T-cell destruction of the self-cell. While this may be beneficial under ordinary circumstances, cancer cells may also express CD80/86 or PD-L1 to prevent their own destruction by the immune system. ICIs have been developed to enhance immune detection and elimination of cancer cells by blocking this interaction [1,2]. GMCSF effectively enhances immune function; the combination of GM-CSF with ICI therapy appears mechanistically synergistic. Indeed, research is underway to evaluate the use of GM-CSF+ICIs in vitro and in vivo.
Combination of GM-CSF with ipilimumab, a CTLA-4 inhibitor, has proven successful in several trials [123]. In one study of advanced ovarian cancer, ipilimumab was administered to patients who had previously received a vaccine transduced with GM-CSF. In this study, patients showed increased inflammatory infiltrate as well as tumor regression, demonstrating improved anticancer activity of the ICI via an immune mechanism [124,125]. Another study evaluated pancreatic cancer patients receiving ipilimumab alone vs. ipilimumab+GVAX. The combination arm uniquely demonstrated a downward trend in CA 19-9 levels, unlike the ipilimumab monotherapy arm. The medial Overall Survival (OS) in the combination group was improved from the ipilimumab monotherapy group (5.7 months vs. 3.6 months, respectively) with enhanced 1-year OS (27% vs. 7%, respectively). Furthermore, significant enhancement of T-cell repertoire was demonstrated (p=0.031) [126].
Additionally, a murine hepatoma model tested the systemic anticancer effects of local GM-CSF microspheres in combination with microwave radiotherapy and anti-CTLA-4 blockade. The mice received various combinations of these treatments, and then were rechallenged with tumor cells 8 weeks after treatment. Following microwave radiation alone, only 20% of mice rejected the tumor rechallenge. Following microwave radiation+GM-CSF, 50% rejected the tumor rechallenge. Finally, 90% of mice who received all 3 (radiation+GM-CSF microspheres +CTLA-4 blockade) rejected the tumor rechallenge. This demonstrates enhanced antitumor immunity in the combination group. This group also saw elimination of distant tumors, despite local injection indicating an abscopal effect [127].
Similar synergism has been identified between GM-CSF and inhibition of the PD-1/PD-L1 axis. One study examined the combination of PD-1 blockade with GM-CSF-secreting tumor cell immunotherapy in mice models of melanoma and colon carcinoma. Interestingly, mice with the combination therapy had improved survival compared with mice receiving either treatment alone. The immune mechanism was validated by several measurements. First, an in vivo CTL assay demonstrated improved antigen-specific T-cell response, which correlated with survival. Second, splenocytes were observed to secrete higher levels of proinflammatory cytokines. Finally, the tumor microenvironment was enriched with functional CD8+ T-lymphocytes [128]. Taken together, these results demonstrate an immune-driven synergism between GM-CSF and ICIs. Subsequently, some mice continued to receive the combination, while others went back to monotherapy with either the PD-1 blockade or the GM-CSF-secreting tumor cell immunotherapy. Interestingly, the improved antigen-specific T-cell expansion only persisted in the combination group [128].
There is some evidence that PD-1/PD-L1 blockade may potentiate the anticancer immune response triggered by GM-CSF vaccines. Researchers found that mice who received GM-CSF demonstrated increased expression of PD-1 by T cells, with increased PD-L1 expression on tumor cells. This group compared PD-1/PDL1 blockade alone, GM-CSF vaccine alone, and PD-1/PD-L1 blockade + GM-CSF vaccine together. The combination produced superior anti-cancer effects compared to either monotherapy, with delayed tumor growth (p<0.05) or decreased tumor weight (p<0.05). Interestingly, the anticancer response was maintained when mice were rechallenged with prostate cancer cells (the same as cell line as the original tumor). The anticancer effects were not seen when rechallenged with a melanoma cell line. This indicates that the generated T-cell response was specific to the original tumor’s antigen [129]. In a phase I/II study patients with colorectal adenocarcinoma were administered GVAX along with cyclophosphamide and pembrolizumab, an anti-PD-1 antibody. Although the phase I/II study was discontinued due to absent primary objective responses, the authors highlighted a significant decline (≥ 30%) in Carcinoembryonic Antigen (CEA) levels, along with increased anti-CEA antibodies in 7 out of 17 patients. As pembrolizumab therapy alone did not affect CEA levels in prior clinical studies, GVAX combined with cyclophosphamide was determined to upregulate an immune response [86]. Taken together, these studies demonstrate enhanced immune-driven anticancer effects when ICIs are enhanced with GM-CSF, and vice versa.
Conclusion
As cancer cells evolve to express novel mutations, so must our cancer therapeutics evolve with novel discoveries and applications? With increased understanding and appreciation of GM-CSF's role in immune modulation, the therapeutic role of GM-CSF is expanding to address the demand for effective immune-based cancer therapeutics. Through the effects of GM-CSF on cell mediators, this signaling molecule influences proliferation and survival of immune cells along with the release of pro-inflammatory cytokines. In addition, the immune system machinery can also be vastly expanded through activation and proliferation of DCs and T cells.
This array of signaling mechanisms can be harnessed for utilization in cancer therapeutics. Recent clinical studies analyzed the efficacy of GM-CSF as a primary therapy and as an adjuvant therapy for chemotherapy, immunotherapy and radiation. With the encouraging results observed, research trials with GM-CSF should continue to increase options for cancer therapy. GM-CSF should also be studied in specific types of cancer to fully evaluate the therapeutic utility as well as the side effects of this therapy. In these analyses, particular attention should be given to concurrent signaling patterns that may influence the efficacy of GM-CSF so that ideal responders can be identified based on the tumor or tumor microenvironment characteristics. Because GM-CSF interacts with a wide array of molecular pathways, additional investigations should be pursued to further reveal details of GM-CSF mechanisms. Together, these observations will guide implementation of GMCSF as an anti-tumor therapeutic agent.
REFERENCES
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms.Mol Cell Biol. 2005;25(21):9543-9553.
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568-571.
- Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer.J Clin Oncol. 2006;24(19):3089-3094.
- Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma.J Clin Oncol. 2009;27(34):5763.
- Kaufman HL, Bines SD. OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future oncol. 2010;6(6):941-949.
- Plosker GL. Sipuleucel-T. Drugs. 2011;71(1):101-108.
- Waldmann TA. Cytokines in cancer immunotherapy.Cold Spring Harb Perspect Biol. 2018;10(12):a028472.
- Kaufman HL, Ruby CE, Hughes T, Slingluff CL. Current status of granulocyte–macrophage colony-stimulating factor in the immunotherapy of melanoma.J Immunother Cancer. 2014;2(1):11.
- Nemunaitis J, Rabinowe SN, Singer JW, Bierman PJ, Vose JM, Freedman AS, et al. Recombinant granulocyte-macrophage colony-stimulating factor after autologous bone marrow transplantation for lymphoid cancer.N Engl J Med. 1991;324(25):1773-8.
- Pharmaceuticals, B.H., sargramastin Package insert, 2020.
- Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy.2016:e1115641.
- Andtbacka RH, Collichio F, Harrington KJ, Middleton MR, Downey G,Ӧhrling K, et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III–IV melanoma.J Immunother Cancer. 2019;7(1):145.
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer.N Engl J Med. 2010;363(5):411-22.
- Sims RB. Sipuleucel-T: autologous cellular immunotherapy for men with asymptomatic or minimally symptomatic metastatic castrate resistant prostate cancer.J Cancer. 2011;2:357.
- Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine.Clin Cancer Res. 2011;17(11):3520-3526.
- Bhattacharya P, Thiruppathi M, Elshabrawy HA, Alharshawi K, Kumar P, Prabhakar BS. GM-CSF: An immune modulatory cytokine that can suppress autoimmunity. Cytokine. 2015;75(2):261-271.
- Van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012;119(15):3383-393.
- Ninck S, Reisser C, Dyckhoff G, Helmke B, Bauer H, Herold‐Mende C. Expression profiles of angiogenic growth factors in squamous cell carcinomas of the head and neck.Int J Cancer. 2003;106(1):34-44.
- Hirata Y, Egea L, Dann SM, Eckmann L, Kagnoff MF. GM-CSF-facilitated dendritic cell recruitment and survival govern the intestinal mucosal response to a mouse enteric bacterial pathogen. Cell host & microbe. 2010;7(2):151-163.
- Hong IS. Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types.Exp Mol Med. 2016;48(7):e242.
- Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell research. 2006;16(2):126-133.
- Furuya MY, Asano T, Sumichika Y, Sato S, Kobayashi H, Watanabe H, et al. Tofacitinib inhibits granulocyte–macrophage colony-stimulating factor-induced NLRP3 inflammasome activation in human neutrophils. J Biol Chem. 2018;20(1):1-9.
- Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651.
- Israël A. The IKK complex, a central regulator of NF-κB activation. Cold Spring Harb Perspect Biol. 2010;2(3):a000158.
- Ebner K, Bandion A, Binder BR, de Martin R, Schmid JA. GMCSF activates NF-κB via direct interaction of the GMCSF receptor with IκB kinase β. Blood. 2003;102(1):192-199.
- Gerondakis S, Siebenlist U. Roles of the NF-κB pathway in lymphocyte development and function. Cold Spring Harb Perspect Biol. 2010;2(5):a000182.
- Sepulveda P, Encabo A, Carbonell-Uberos F, Minana MD. BCL-2 expression is mainly regulated by JAK/STAT3 pathway in human CD34+ hematopoietic cells. Cell Death Differ. 2007;14(2):378-380.
- Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117(8):1281-1283.
- Brutkiewicz RR. Cell signaling pathways that regulate antigen presentation. J Immunol. 2016 ;197(8):2971-2979.
- Osborn JL, Greer SF. Metastatic melanoma cells evade immune detection by silencing STAT1. Int J Mol Sci. 2015;16(2):4343-4361.
- Van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012;119(15):3383-3393.
- Chousterman BG, Arnaud M. Is there a role for hematopoietic growth factors during sepsis?. Front Immunol. 2018;9:1015.
- Mroczko B, Szmitkowski M. Hematopoietic cytokines as tumor markers. Clin Chem Lab Med (CCLM). 2004;42(12):1347-1354.
- Nebiker CA, Han J, Eppenberger-Castori S, Iezzi G, Hirt C, Amicarella F, et al. GM-CSF production by tumor cells is associated with improved survival in colorectal cancer. Clin Cancer Res. 2014;20(12):3094-3106.
- Revoltella RP, Menicagli M, Campani D. Granulocyte–macrophage colony-stimulating factor as an autocrine survival-growth factor in human gliomas. Cytokine. 2012;57(3):347-359.
- Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. 2018;172(1-2):147-161.
- Chicha L, Feki A, Boni A, Irion O, Hovatta O, Jaconi M. Human pluripotent stem cells differentiated in fully defined medium generate hematopoietic CD34+ and CD34: Progenitors with distinct characteristics. PLoS One. 2011;6(2):e14733.
- Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28(5):509-554.
- Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 2013;34(2):81-89.
- Nagdy B, Kassem HA, Abdel-Ghaffar AR, Seoudi DM, Kassem NM. The Clinicopathological Impact of Granulocyte-Macrophage Colony-Stimulating Factor Gene Expression and Different Molecular Prognostic Biomarkers in Egyptian Acute Myeloid Leukemia Patients. J Glob Oncol: APJCP. 2020;21(7):1993.
- Rosas M, Gordon S, Taylor PR. Characterisation of the expression and function of the GM‐CSF receptor α‐chain in mice. Eur J Immunol. 2007;37(9):2518-2528.
- Till KJ, Burthem J, Lopez A, Cawley JC. Granulocyte-macrophage colony-stimulating factor receptor: stage-specific expression and function on late B cells.1996: 479-486.
- Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71(5):357-370.
- Guo X, Higgs BW, Bay-Jensen AC, Wu Y, Karsdal MA, Kuziora M, et al. Blockade of GM-CSF pathway induced sustained suppression of myeloid and T cell activities in rheumatoid arthritis. Rheumatol. 2018;57(1):175-184.
- Bowne WB, Wolchok JD, Hawkins W, Srinivasan R, Gregor P, Blachere NE, et al. Injection of DNA encoding granulocyte-macrophage colony-stimulating factor recruits dendritic cells for immune adjuvant effects. Cytokines Cell Mol Ther. 1999;5(4):217-225.
- Hiasa M, Abe M, Nakano A, Oda A, Amou H, Kido S, et al. GM-CSF and IL-4 induce dendritic cell differentiation and disrupt osteoclastogenesis through M-CSF receptor shedding by up-regulation of TNF-α converting enzyme (TACE). Blood. 2009;114(20):4517-4526.
- Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160(9):4587-4595.
- Chapuis F, Rosenzwajg M, Yagello M, Ekman M, Biberfeld P, Gluckman JC. Differentiation of human dendritic cells from monocytes in vitro. Eur J Immunol. 1997 Feb;27(2):431-441.
- Becher B, Tugues S, Greter M. GM-CSF: from growth factor to central mediator of tissue inflammation. Immunity. 2016;45(5):963-973.
- Ferenbach D, Hughes J. Macrophages and dendritic cells: what is the difference?. Kidney int. 2008;74(1):5-7.
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nature Rev Immunol. 2007;7(1):19-30.
- Merad M, Manz MG. Dendritic cell homeostasis. Blood,. 2009;113(15):3418-3427.
- Gillessen S, Naumov YN, Nieuwenhuis EE, Exley MA, Lee FS, Mach N, et al. CD1d-restricted T cells regulate dendritic cell function and antitumor immunity in a granulocyte–macrophage colony-stimulating factor-dependent fashion. Proceedings of the National Academy of Sciences. 2003;100(15):8874-8879.
- Borràs FE, Matthews NC, Lowdell MW, Navarrete CV. Identification of both myeloid CD11c+ and lymphoid CD11c− dendritic cell subsets in cord blood. Br J Haematol. 2001;113(4):925-931.
- Steinman RM, Inaba K. Myeloid dendritic cells. J Leukoc Biol. 1999;66(2):205-208.
- McDermott AJ, Frank CR, Falkowski NR, McDonald RA, Young VB, Huffnagle GB. Role of GM-CSF in the inflammatory cytokine network that regulates neutrophil influx into the colonic mucosa during Clostridium difficile infection in mice. Gut microbes. 2014;5(4):10-19.
- Jarmin DI, Nibbs RJ, Jamieson T, de Bono JS, Graham GJ. Granulocyte macrophage colony-stimulating factor and interleukin-3 regulate chemokine and chemokine receptor expression in bone marrow macrophages. Exp Hematol. 1999;27(12):1735-1745.
- Tiberio L, Del Prete A, Schioppa T, Sozio F, Bosisio D, Sozzani S. Chemokine and chemotactic signals in dendritic cell migration. Cell Mol Immunol. 2018;15(4):346-352.
- Ji Q, Gondek D, Hurwitz AA. Provision of granulocyte-macrophage colony-stimulating factor converts an autoimmune response to a self-antigen into an antitumor response. J Immunol. 2005;175(3):1456-1463.
- Gonzalez‐Juarrero M, Hattle JM, Izzo A, Junqueira‐Kipnis AP, Shim TS, Trapnell BC, et al. Disruption of granulocyte macrophage‐colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol. 2005;77(6):914-922.
- Selvarajan V, Bidkar AP, Shome R, Banerjee A, Chaubey N, Ghosh SS, et al. Studying in vitro phagocytosis of apoptotic cancer cells by recombinant GMCSF-treated RAW 264.7 macrophages. Int J Biol Macromol. 2017;102:1138-1145.
- Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P, et al. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity. 2015;43(3):502-514.
- Boyette LB, Macedo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, et al. Phenotype, function, and differentiation potential of human monocyte subsets. PloS one. 2017;12(4):e0176460.
- Swacha P, Gekara NO, Erttmann SF. Biochemical and microscopic analysis of inflammasome complex formation. Meth Enzymol. 2019;625:287-298.
- Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. . J Immunol. 2001;166(4):2717-2726.
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99-109.
- Lu L, McCaslin D, Starzl TE, Thomson AW. Bone marrow–derived dendritic cell progenitors (NLDC 145+, MHC CLASS II+, B7–1dim, B7–2−) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation. 1995;60(12):1539.
- Lu L, Woo J, Rao AS, Li Y, Watkins SC, Qian S, et al. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J Exp Med. 1994 ;179(6):1823-1834.
- Voskamp AL, Prickett SR, Mackay F, Rolland JM, O'Hehir RE. MHC class II expression in human basophils: induction and lack of functional significance. PLoS One. 2013;8(12):e81777.
- Hornell TM, Beresford GW, Bushey A, Boss JM, Mellins ED. Regulation of the class II MHC pathway in primary human monocytes by granulocyte-macrophage colony-stimulating factor. J Immunol. 2003;171(5):2374-2383.
- Nemeckova S, Smahel M, Hainz P, Mackova I, Zurkova K, Gabriel P, et al. Combination of intratumoral injections of vaccinia virus MVA expressing GM-CSF and immunization with DNA vaccine prolongs the survival of mice bearing HPV16 induced tumors with downregulated expression of MHC class I molecules. Neoplasma. 2007;54(4):326.
- Klagge I, Kopp U, Koch N. Granulocyte–macrophage colony‐stimulating factor elevates invariant chain expression in immature myelomonocytic cell lines. Immunol. 1997 ;91(1):114-120.
- Lotfi N, Thome R, Rezaei N, Rezaei A, Rostami A, Esmaeil N. Roles of GM-CSF in the pathogenesis of autoimmune diseases: an update. Front Immunol. 2019;10:1265.
- Rasouli J, Ciric B, Imitola J, Gonnella P, Hwang D, Mahajan K, Mari ER, Safavi F, Leist TP, Zhang GX, Rostami A. Expression of GM-CSF in T cells is increased in multiple sclerosis and suppressed by IFN-β therapy. J Immunol. 2015 Jun 1;194(11):5085-5093.
- Barouch DH, Santra S, Tenner-Racz K, Racz P, Kuroda MJ, Schmitz JE, Jackson SS, Lifton MA, Freed DC, Perry HC, Davies ME. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. . J Immunol. 2002;168(2):562-568.
- Høglund RA, Maghazachi AA. Multiple sclerosis and the role of immune cells. World J Exp Med. 2014;4(3):27.
- Tso CL, Zisman A, Pantuck A, Calilliw R, Hernandez JM, Paik S,et al. Induction of G250-targeted and T-cell-mediated antitumor activity against renal cell carcinoma using a chimeric fusion protein consisting of G250 and granulocyte/monocyte-colony stimulating factor. Cancer res. 2001;61(21):7925-7933.
- Kantoff PW, Higano CS, Small EJ, Whitmore JB, Frohlich MW, Schellhammer PF. Re: interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst. 2012;104(14):1107-1109.
- Lawson DH, Lee S, Zhao F, Tarhini AA, Margolin KA, Ernstoff MS, et al. Randomized, placebo-controlled, Phase III trial of yeast-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) versus peptide vaccination versus GM-CSF plus peptide vaccination versus placebo in patients with no evidence of disease after complete surgical resection of locally advanced and/or stage IV melanoma: a trial of the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group (E4697). J Clin Oncol. 2015;33(34):4066.
- Ng J, Dai T. Radiation therapy and the abscopal effect: A concept comes of age. Ann Transl Med. 2016;4(6).
- Craig DJ. The Abscopal Effect of Radiation Therapy. Future Oncology, 2020.
- Yilmaz MT, Elmali A, Yazici G. Abscopal effect, from myth to reality: from radiation oncologists' perspective. Cureus. 2019;11.
- Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16(7):795-803.
- Leary R, Gardner RB, Mockbee C, Roychowdhury DF. Boosting abscopal response to radiotherapy with sargramostim: A review of data and ongoing studies. Cureus. 2019;11: e4276.
- Nemunaitis J, Jahan T, Ross H, Sterman D, Richards D, Fox B,et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX® vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther. 2006;13(6):555-562.
- Yarchoan M, Huang CY, Zhu Q, Ferguson AK, Durham JN, Anders RAet al. A phase 2 study of GVAX colon vaccine with cyclophosphamide and pembrolizumab in patients with mismatch repair proficient advanced colorectal cancer. Cancer med. 2020;9(4):1485-1494.
- Yan WL, Shen KY, Tien CY, Chen YA, Liu SJ. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy. 2017;9(4):347-360.
- Cassidy T, Craig M. Determinants of combination GM-CSF immunotherapy and oncolytic virotherapy success identified through in silico treatment personalization. PLoS Comput Biol. 2019;15(11):e1007495.
- Spitler LE, Cao H, Piironen T, Whiteside TL, Weber RW, Cruickshank S. Biologic Effects of Anti-Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Antibody Formation in Patients Treated with GM-CSF (Sargramostim) as Adjuvant Therapy of Melanoma. Am J Clin Oncol. 2017;40(2):207.
- Butterfield LH, Zhao F, Lee S, Tarhini AA, Margolin KA, White RL, et al. Immune correlates of GM-CSF and melanoma peptide vaccination in a randomized trial for the adjuvant therapy of resected high-risk melanoma (E4697). Clin Cancer Res. 2017 Sep 1;23(17):5034-5043.
- Lilleby W, Gaudernack G, Brunsvig PF, Vlatkovic L, Schulz M, Mills K, et al. Phase I/IIa clinical trial of a novel hTERT peptide vaccine in men with metastatic hormone-naive prostate cancer. Cancer Immunol Immunother. 2017;66(7):891-901.
- Lipson EJ, Sharfman WH, Chen S, McMiller TL, Pritchard TS, Salas JT, et al. Safety and immunologic correlates of Melanoma GVAX, a GM-CSF secreting allogeneic melanoma cell vaccine administered in the adjuvant setting. J Transl Med. 2015;13(1):1-4.
- Curry WT, Gorrepati R, Piesche M, Sasada T, Agarwalla P, Jones PS, et al. Vaccination with irradiated autologous tumor cells mixed with irradiated GM-K562 cells stimulates antitumor immunity and T lymphocyte activation in patients with recurrent malignant glioma. Clin Cancer Res. 2016;22(12):2885-2896.
- Vuky J, Corman JM, Porter C, Olgac S, Auerbach E, Dahl K. Phase II trial of neoadjuvant docetaxel and CG1940/CG8711 followed by radical prostatectomy in patients with high-risk clinically localized prostate cancer. Oncologist. 2013;18(6):687.
- Higano C. A phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, Castration-Resistant Prostate Cancer (CRPC). InProceedings of the 2009 Genitourinary Cancer Symposium, J Clin Oncol.2009:26-28,
- Small EJ. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel versus docetaxel plus prednisone in symptomatic, Castration-Resistant Prostate Cancer (CRPC). InProceedings of the 2009 Genitourinary Cancer Symposium, American Society of Clinical Oncology (ASCO), Orlando, FL, USA. 2009: 26-28.
- Nemunaitis J, Murray N. Immune-modulating vaccines in non-small cell lung cancer. J Thorac Oncol. 2006 Sep 1;1(7):756-761.
- Nemunaitis J, Sterman D, Jablons D, Smith JW, Fox B, Maples P, et al. Granulocyte–macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non–small-cell lung cancer. J Natl Cancer Inst. 2004;96(4):326-331.
- Nemunaitis J. GVAX (GMCSF gene modified tumor vaccine) in advanced stage non-small cell lung cancer. J Control Release. 2003;91(1-2):225-231.
- Ferguson MS, Lemoine NR, Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv Virol.2012.
- Malhotra S, Kim T, Zager J, Bennett J, Ebright M, D’Angelica M, et al. Use of an oncolytic virus secreting GM-CSF as combined oncolytic and immunotherapy for treatment of colorectal and hepatic adenocarcinomas. Surgery. 2007;141(4):520-529.
- Bristol JA, Zhu M, Ji H, Mina M, Xie Y, Clarke L, et al. In vitro and in vivo activities of an oncolytic adenoviral vector designed to express GM-CSF. Mol Ther. 2003;7(6):755-764.
- Deng L, Fan J, Guo M, Huang B. Oncolytic and immunologic cancer therapy with GM-CSF-armed vaccinia virus of Tian Tan strain Guang9. Cancer Lett. 2016;372(2):251-257.
- De Matos AL, Franco LS, McFadden G. Oncolytic viruses and the immune system: The dynamic duo. Mol Ther Methods Clin Dev. 2020;17:349-358.
- Johnson DG, Schneider-Broussard R. Role of E2F in cell cycle control and cancer. Front Biosci. 1998;3:d447-d448.
- Hemminki O, Parviainen S, Juhila J, Turkki R, Linder N, Lundin J, et al. Immunological data from cancer patients treated with Ad5/3-E2F-Δ24-GMCSF suggests utility for tumor immunotherapy. Oncotarget. 2015;6(6):4467.
- Melcher A, Parato K, Rooney CM, Bell JC. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther. 2011;19(6):1008-1016.
- Maples PB, Kumar PA, Yu Y, Wang Z, Jay C, Pappen BO, et al. FANG vaccine: autologous tumor cell vaccine genitically modified to express GM-CSF and block production of Furin. BioProcessing Journal. 2010;8:4-14.
- De Matos AL, Franco LS, McFadden G. Oncolytic viruses and the immune system: The dynamic duo. Mol Ther Methods Clin Dev. 2020;17:349-358.
- Senzer N, Barve M, Kuhn J, Melnyk A, Beitsch P, Lazar M et al. Phase I trial of “bi-shRNAifurin/GMCSF DNA/autologous tumor cell” vaccine (FANG) in advanced cancer. Mol Ther. 2012;20(3):679-686.
- Senzer N, Barve M, Nemunaitis J, Kuhn J, Melnyk A, Beitsch P, et al. Long term follow up: Phase I trial of “bi-shRNA furin/GMCSF DNA/autologous tumor cell” immunotherapy (FANG™) in advanced cancer. J Vaccines Vaccin. 2013;4(209):10-4172.
- Barve M, Kuhn J, Lamont J, Beitsch P, Manning L, Pappen BO, et al. Follow-up of bi-shRNA furin/GM-CSF engineered autologous tumor cell (EATC) immunotherapy Vigil in patients with advanced melanoma. Biomed. Genet. Genomics. 2016;1(3):81-86.
- Oh J, Barve M, Matthews CM, Koon EC, Heffernan TP, Fine B, Grosen E, Bergman MK, Fleming EL, DeMars LR, West L. Phase II study of Vigil DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol Oncol. 2016;143(3):504-510.
- Ghisoli M, Barve M, Schneider R, Mennel R, Lenarsky C, Wallraven G, et al. Pilot trial of FANG immunotherapy in Ewing's sarcoma. Mol Ther. 2015;23(6):1103-1109.
- Ghisoli M, Manning L, Senzer N, Nemunaitis J. Innovative exploratory clinical approaches for relapsed and/or refractory metastatic Ewing's sarcoma. Clin. Oncol. 2016;1:1079.
- Ghisoli M, Barve M, Mennel R, Lenarsky C, Horvath S, Wallraven G, et al. Three-year follow up of GMCSF/bi-shRNAfurin DNA-transfected Autologous tumor immunotherapy (vigil) in metastatic advanced Ewing's sarcoma. Mol Ther. 2016;24(8):1478-1483.
- Herron J, Smith N, Stanbery L, Aaron P, Manning L, Bognar E, et al. Vigil: Personalized Immunotherapy Generating Systemic Cytotoxic T cell Response. Cancer Sci. Res. 2020;1:210-221.
- Ghisoli M, Rutledge M, Stephens PJ, Mennel R, Barve M, Manley M, et al. Case report: immune-mediated complete response in a patient with recurrent advanced Ewing sarcoma (EWS) after vigil immunotherapy. J Pediatr Hematol Oncol. 2017;39(4):e183-e186.
- Oh J, Barve M, Senzer N, Aaron P, Manning L, Wallraven G, Bognar E, et al. Long-term follow-up of Phase 2A trial results involving advanced ovarian cancer patients treated with Vigil® in frontline maintenance. Gynecol Oncol Rep. 2020:100648.
- Oh J, Barve M, Matthews CM, Koon EC, Heffernan TP, Fine B, et al. Phase II study of Vigil® DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol Oncol. 2016;143(3):504-510.
- McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al, Watkins TB. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463-1469.
- Morand S, Stanbery L, Walter A, Rocconi RP, Nemunaitis J. BRCA1/2 mutation status impact on autophagy and immune response: Unheralded target. JNCI Cancer Spec. 2020.
- Heong V, Ngoi N, Tan DS. Update on immune checkpoint inhibitors in gynecological cancers. J Gynecol Oncol.. 2016;28(2).
- Rocconi RP, Grosen EA, Ghamande SA, Chan JK, Barve MA, Oh J, et al. Randomized double-blind placebo-controlled trial of primary maintenance vigil immunotherapy (VITAL study) in stage III/IV ovarian cancer: Efficacy assessment in BRCA1/2-wt patients.2020:6017.
- Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci. 2008;105(8):3005-3010.
- Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci. 2003;100(8):4712-4717.
- Chen Z, Shen S, Peng B, Tao J. Intratumoural GM-CSF microspheres and CTLA-4 blockade enhance the antitumour immunity induced by thermal ablation in a subcutaneous murine hepatoma model. Int J Hyperthermia. 2009;25(5):374-382.
- Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36(7):382.
- Li B, VanRoey M, Wang C, Chen TH, Korman A, Jooss K. Anti–programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor–secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15(5):1623-1634.
Citation: Morand S, Devanaboyina M, Fung C, Royfman R, Filipiak L, Stanbery L, et al. (2020) GM-CSF: Anti-Cancer Immune Response and Therapeutic Application. J Vaccines Vaccin. S10: 002.
Copyright: © 2020 Morand S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.