Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2023) Volume 14, Issue 3
Genotyping of Blood Donors for Timely Procurement of Rare Antigen-Negative Blood: A Case Story of Acute HTR and Anti-Coa
Katrine Kielsen1, Bibi Fatima Syed Shah Scharff1, Anne Todsen Hansen1, Line Malmer Madsen2, Niels Erikstrup Clausen2, Klaus Rieneck1 and Morten Hanefeld Dziegiel1,3*2Department of Anaesthesia and Intensive Care, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Copenhagen, Denmark
3Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
Received: 08-Feb-2023, Manuscript No. JBDT-23-19764; Editor assigned: 10-Feb-2023, Pre QC No. JBDT-23-19764 (PQ); Reviewed: 03-Mar-2023, QC No. JBDT-23-19764; Revised: 10-Mar-2023, Manuscript No. JBDT-23-19764 (R); Published: 17-Mar-2023, DOI: 10.4172/2155-9864.23.14.553
Abstract
Hemolytic Transfusion Reactions (HTR) caused by antibodies against the high-prevalence Coa antigen has rarely been reported, and Coa-negative Red Blood Cells (RBC) units are rare.
A 74-year-old woman was admitted with gastro-intestinal bleeding and sepsis. She developed an alloantibody of unknown specificity after her first transfusion. After a third, urgent transfusion with a Coa incompatible RBC unit, she reacted with dyspnea, hypertension, cerebral impairment and ultimately cardiac arrest. Hemolysis was demonstrated, an anti-Coa was detected in her serum, and post transfusion investigations identified the implicated RBC unit as Co (a+b-). In the following weeks, the patient’s persistent need for RBC transfusions was met by fresh Coa-negative blood enabled by our recently implemented routine genotyping of blood donors. Genotyping of the patient’s blood group antigens was an important diagnostic tool in the identification of the antibody, and large-scale genotyping of blood donors proved to be of great value to procure Coa negative blood.
Keywords
Colton-a; Alloantibody; Genotyping; Acute hemolytic transfusion reaction
Introduction
The Colton (co) blood group antigens are located on aquaporin-1, a water channel protein regulating water homeostasis in erythrocytes and kidney tubules [1]. The highprevalence antigen Coa is present in 99.8% of Caucasians, while the antithetical antigen Cob is reported in 8.5% and most frequently caused by a single-nucleotide polymorphism introducing an alanine to valine substitution [2-4].
Anti-Coa antibodies were first described by Heisto, et al. in 1967 [5]. They are most commonly of IgG isotype and formed after exposure of a Coa negative individual to antigen-positive blood by transfusion or pregnancy [2, 4]. Antibodies against Coa have been described to cause severe Hemolytic Disease of the Fetus and Newborn (HDFN), but publications of Hemolytic Transfusion Reactions (HTR) are rare [6-9]. Here, we report a case of severe acute HTR associated with development of an anti- Coa antibody and the subsequent attempts to provide Co (a-) blood products to the patient.
Case Presentation
A 74-year-old Caucasian woman with a prior medical history of hysterectomy, ovariectomy and breast cancer and currently undergoing tamoxifen treatment for invasive ductal carcinoma was admitted to the hospital with abdominal pain (Figure 1). She was diagnosed with acute diverticulitis and treated with intravenous antibiotics but progressed with intestinal necrosis and perforation after 11 days. A subtotal colectomy and resection of the small intestines were performed. Postoperatively, she was transferred to the Intensive Care Unit (ICU) with septic shock. Escherichia coli and Enterococcus faecium were identified in ascitic fluid, and E. faecium was also detected in blood culture. She received ventilatory support and broad-spectrum antibiotics with clinical improvement and normalization of infectious parameters, but increasing signs of anemia.
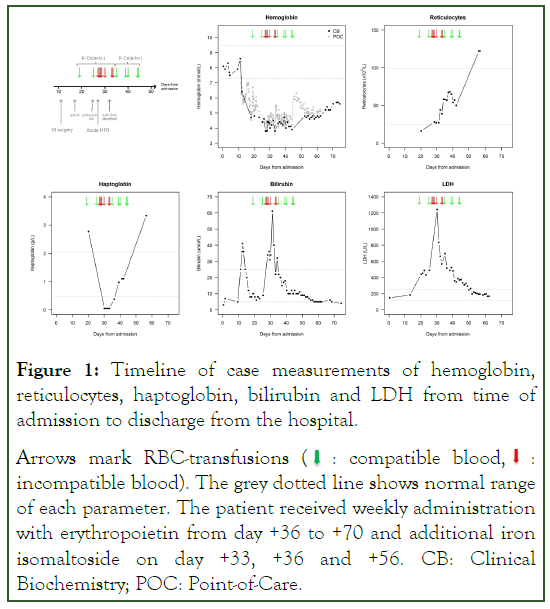
Figure 1: Timeline of case measurements of hemoglobin,
reticulocytes, haptoglobin, bilirubin and LDH from time of
admission to discharge from the hospital.
Arrows mark RBC-transfusions (  : compatible blood,
: compatible blood,  :
incompatible blood). The grey dotted line shows normal range
of each parameter. The patient received weekly administration
with erythropoietin from day +36 to +70 and additional iron
isomaltoside on day +33, +36 and +56. CB: Clinical
Biochemistry; POC: Point-of-Care.
:
incompatible blood). The grey dotted line shows normal range
of each parameter. The patient received weekly administration
with erythropoietin from day +36 to +70 and additional iron
isomaltoside on day +33, +36 and +56. CB: Clinical
Biochemistry; POC: Point-of-Care.
Her transfusion history was limited to a single RBC unit 13 years earlier. Initial testing identified her as blood group B RhD+ with negative antibody screen tests before and two days after surgery. Despite the absence of transfusion, a weak anti-K antibody was detected by panel antibody investigation Indirect Antiglobulin Test (IAT technique) six days post-operative. Accordingly, the patient’s own RBCs were identified as K-negative by serological phenotyping (Bio-Rad gel column agglutination system). Direct Antiglobulin Test (DAT) was negative, and the patient received a single compatible K-negative RBC unit.
Fourteen days after this transfusion, following reassignment to the gastrointestinal ward, an additional alloantibody of unknown specificity developed. A second K-negative RBC unit with full serological compatibility (IAT) was administered, but for the following two days no compatible units could be identified despite intensive efforts. Meanwhile, the patient’s hemoglobin level decreased, and atrial flutter arose. A third transfusion with the best compatible K-negative RBC unit was given despite a serological crossmatch of +1 (IAT). After infusion of approximately half of the product, the patient developed dyspnea, desaturation (80%), hypertension (BP 200/100), chills, and cerebral impairment. She was treated with 40 mg furosemide intravenously and continuous positive airway pressure on suspicion of pulmonary edema with all symptoms resolving. An investigation for hemolytic transfusion reaction was started. Four hours after the transfusion, the patient was found unresponsive with tachypnea (RR 24), desaturation (86%with 14 L oxygen) and hypotension (BP 59/34), shortly afterwards developing cardiac arrest. She received cardiopulmonary resuscitation, was converted back to normal sinus rhythm and readmitted to the ICU. Her clinical condition deteriorated with septic shock from multiple intraabdominal abscesses and disseminated intravascular coagulation.
Despite transfusion, hemoglobin declined from 4.6 mmol/L (7.4 g/dL) to 3.8 mmol/L (6.1 g/dL) with no clinical evidence of bleeding. Blood samples collected shortly after the transfusion showed a positive DAT with 1+ for C3d, but no reaction for Immunoglobulin G (IgG). Initial antibody identification showed reactions of 1+ to 3+ with 10 of 11 of the panel cells by IAT with Polyethylene Glycol (PEG), and reactions decreased with papain and Dithiothreitol (DTT) treatment (saline without PEG). Additional blood samples collected during the next two days reacted with all RBCs in an unspecific pattern (Figure 2). Supplementary RBC phenotyping was challenged by prior blood transfusions. However, blood group genotyping using RBCFluoGene verify Extend (Inno-Train, Kronberg, Germany) revealed the infrequent CO*O2 genotype of the patient corresponding to a Co (a-b+) phenotype. Re-evaluation of the antibody panel indicated an initial dosage effect arising from heterozygosity in the Colton blood group, which were partly concealed by the anti-K antibody and disappeared after the second RBC transfusion. The presence of anti-Coa was proven by compatibility with K-, Co (a-) RBCs from two donors, and non-compatibility with K-Co (a+b-) RBCs from 3 donors (by IAT).
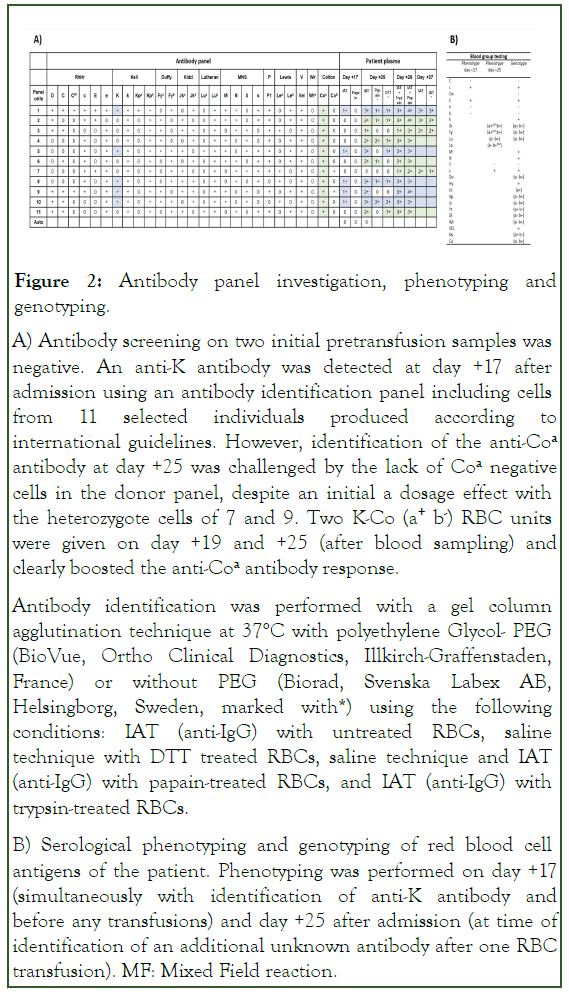
Figure 2: Antibody panel investigation, phenotyping and genotyping.
A) Antibody screening on two initial pretransfusion samples was negative. An anti-K antibody was detected at day +17 after admission using an antibody identification panel including cells from 11 selected individuals produced according to international guidelines. However, identification of the anti-Coa antibody at day +25 was challenged by the lack of Coa negative cells in the donor panel, despite an initial a dosage effect with the heterozygote cells of 7 and 9. Two K-Co (a+ b-) RBC units were given on day +19 and +25 (after blood sampling) and clearly boosted the anti-Coa antibody response.
Antibody identification was performed with a gel column agglutination technique at 37°C with polyethylene Glycol- PEG (BioVue, Ortho Clinical Diagnostics, Illkirch-Graffenstaden, France) or without PEG (Biorad, Svenska Labex AB, Helsingborg, Sweden, marked with*) using the following conditions: IAT (anti-IgG) with untreated RBCs, saline technique with DTT treated RBCs, saline technique and IAT (anti-IgG) with papain-treated RBCs, and IAT (anti-IgG) with trypsin-treated RBCs.
B) Serological phenotyping and genotyping of red blood cell antigens of the patient. Phenotyping was performed on day +17 (simultaneously with identification of anti-K antibody and before any transfusions) and day +25 after admission (at time of identification of an additional unknown antibody after one RBC transfusion). MF: Mixed Field reaction.
Reevaluation revealed that the patients had initially beentransfused with 2 RBC units from K-, Co (a+b-) donors. The third RBC unit causing the transfusion reaction was also later identified as K-, Co (a+b-). Four days later, DAT was positive with 1+ for both IgG and C3d. In the meantime, the patient had received additionally four K-,Co (a+b-) incompatible RBC units, and results from clinical biochemistry analyses showed signs of severe hemolysis (Figure 1). Based on these findings, acute Hemolytic Transfusion Reactions (HTR) was diagnosed.
Simultaneously, a search for K-, Co (a-) blood donors of blood group B or O was initiated. In our institution, approximately 14,000 (25%) of the active blood donors had been routinely genotyped with a Kompetitive Allele-Specific Polymerase (KASP) assay targeting 16 blood group systems, including Coa identified by Single Nucleotide Polymorphisms (SNP) rs2836269210. Of the genotyped donors, 0.21% were Co (a-b+), while 8.90% were Co (a+b+) and 90.88% were Co (a+b-), corresponding to allele frequencies of 0.9533 (a-allele) and 0.0467 (b-allele). This timely effort allowed us to procure crucial K-, Co (a-b+) compatible products within three days, and in total RBC units from five available, compatible donors were given. Furthermore, while international request for K-, Co(a-b+) units were made, 10 available RBC units from K-negative and Co(a+b+) heterozygote donors were tested by serological cross matching and surprisingly found fully compatible with the patient and stored in case of emergency.
Transfusion with K-, Co (a-b+) RBC products resulted in gradual normalization of hemolysis parameters within the following three weeks. At the same time patient blood management was initiated parallel to blood transfusion by administration of vitamin B12, folate, erythropoietin and iron isomaltoside based on laboratory results, in order to support the patient’s own erythrocyte production.
The patient remained at the ICU for more than a month and was finally discharged from the hospital 2.5 months after her initial admission.
Results and Discussion
We report a severe acute HTR caused by anti-Coa antibodies, which occurred after transfusion with three Co (a+b-) RBC units and was suspected to cause cardiac arrest of the patient. Antibodies to Coa have in few prior cases been implicated in transfusion reactions ranging from mild delayed hemolytic reactions to acute hemolysis and lifethreatening cases of HDFN [6-11]. In our case, the diagnosis of acute HTR was based on hemolysis immediately after transfusion combined with an incompatible crossmatch. The conclusion was strengthened by the subsequent demonstration of circulating anti-Coa antibodies, the identification of the incompatible RBC as being Co (a+b-), and the lack of hemolysis after transfusions with Coa negative RBC units. Although the patient initially presented with development of an anti-K alloantibody, possibly caused by the sepsis with E. faecium, this anti-K antibody had no role in the transfusion reaction as all transfused RBC units were K-negative [12,13].
This clinical case illustrates that genotyping of red blood group antigens can be an important diagnostic tool in the identification of rare alloantigens. Our serological analyses were challenged by the lack of antigen negative cells and the coinciding presence of an anti-K, partly masking the reactions of Co (a+b+) heterozygous cells in the panel. Patient genotyping, however, revealed the CO*O2 genotype corresponding to the Co(a-b+) phenotype, which provided crucial information to guide the identification of an anti-Coa antibody by testing of patient serum with Co(a-) and Co(a+) RBCs [14].
This case also demonstrates the importance of a large pool of well-typed blood donors in the procurement of compatible blood in cases of rare alloantibodies. The patient required multiple RBC transfusions for two weeks after the identification of the anti-Coa. We managed to collect all the necessary Coanegative units locally, even though the frequency of Co (a-b+) donors was only 0.21% in our population (in line with previous reports) [2, 3].
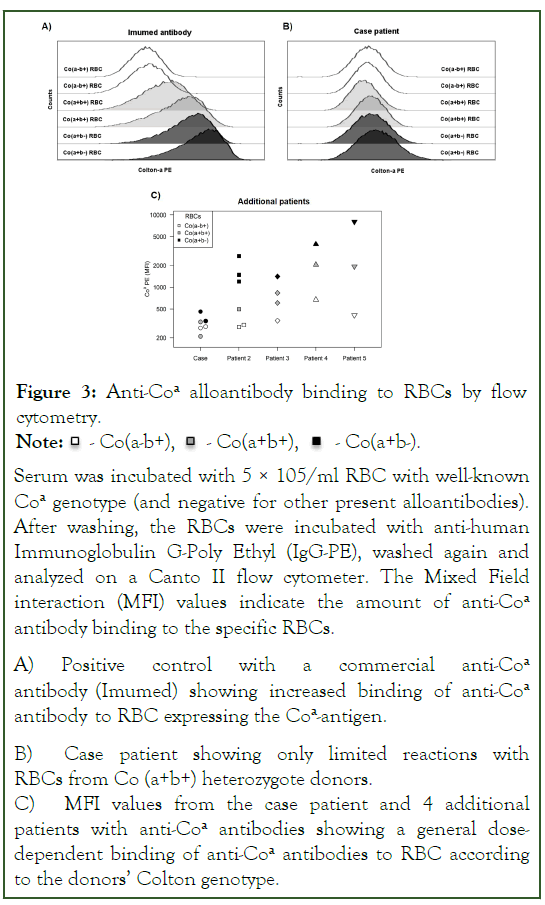
Interestingly, full compatibility was shown between patient serum and ten different Co (a+b+) heterozygous donors by IAT technique. To investigate this further, we tested serum from 5 patients with anti-Coa antibodies against RBCs with different Colton genotypes by flow cytometry and found a general dose dependent response (Figure 3). Similar data has not been presented previously. These data suggest that in some cases, transfusions with heterozygous donors could be used in urgent situations with anti-Coa antibodies, when transfusions cannot await procurement of antigen-negative blood.
Figure 3: Anti-Coa alloantibody binding to RBCs by flow cytometry.
Note: -  Co(a-b+),
Co(a-b+), - Co(a+b+),
- Co(a+b+),  - Co(a+b-).
- Co(a+b-).
Serum was incubated with 5 × 105/ml RBC with well-known Coa genotype (and negative for other present alloantibodies). After washing, the RBCs were incubated with anti-human Immunoglobulin G-Poly Ethyl (IgG-PE), washed again and analyzed on a Canto II flow cytometer. The Mixed Field interaction (MFI) values indicate the amount of anti-Coa antibody binding to the specific RBCs.
A) Positive control with a commercial anti-Coa antibody (Imumed) showing increased binding of anti-Coa antibody to RBC expressing the Coa-antigen.
B) Case patient showing only limited reactions with RBCs from Co (a+b+) heterozygote donors.
C) MFI values from the case patient and 4 additional patients with anti-Coa antibodies showing a general dosedependent binding of anti-Coa antibodies to RBC according to the donors’ Colton genotype.
Conclusion
In conclusion, we report a severe acute HTR in a patient with the rare anti-Coa alloantibody. Genotyping of the patient aided in the identification of the antibody. Furthermore, this case illustrates how large-scale genotyping of blood donors can be crucial in procurement of antigen-negative RBC units to patients with antibodies against frequent antigens and in urgent need for transfusions. In the aftermath of this event, our genotyping of blood donors has led to the establishment of a local inventory of frozen RBCs of several rare phenotypes from our blood donors in case of future needs.
Acknowledgements
KK, BFSSS, KR and MHD performed serological analysis for identification of the antibody and procured compatible blood. LMM and NEC treated the patient and provided clinical expertise. ATH performed the flow cytometry analyses. KK drafted the manuscript. All authors were involved in ongoing discussion of results and revision of the manuscript.
We thank Katri Haimila (Finnish Red Cross Blood Service, Helsinki, Finland) for providing serum from two additional patients with anti-Coa antibodies for further investigations.
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report.
Conflict of Interest Statement
None to report.
Funding Sources
No financial support was obtained for this case report.
References
- Smith BL, Preston GM, Spring FA, Anstee DJ, Agre P. Human Red Cell aquaporin CHIP. I. Molecular characterization of ABH and Colton blood group antigens. J Clin Invest. 1994; 94:1043–1049.
[Crossref] [Google Scholar] [PubMed]
- Daniels G. Colton blood group system. In: Human blood groups. Blackwell Sci. 2002;2: 398–403.
- Lewis M, Kaita H, Chown B, Giblett ER, Anderson J. Colton blood groups in canadian caucasians: Frequencies, inheritance and linkage analysis. Vox Sang. 1977; 32:208–213.
[Crossref] [Google Scholar] [PubMed]
- Halverson GR, Peyrard T. A review of the Colton blood group system. Immunohematology. 2010;26:22–26.
[Crossref] [Google Scholar] [PubMed]
- Heisto H, van der Hart M, Madsen G, Moes M, Noades J, Pickles MM, et al. Three Examples of a New Red Cell Antibody, Anti‐Coa. Vox Sang. 1967;12:18–24.
- Michalewska B, Wielgos M, Zupanska B, Bartkowiak R. Anti-Co a implicated in severe haemolytic disease of the foetus and newborn. Transfus Med. 2008;18:71–73.
- Kimmich N, Brand B, Hustinx H, Komarek A, Zimmermann R. Management of anti-Colton a alloimmunisation in pregnancy: a case report. Transfus Med. 2016;26:150–152.
- Kitzke H, Julius H, Delaney M, Studnicka L, Landmark J. Anti-Coa implicated in delayed hemolytic transfusion reactiont. Transfusion. 1982;22:407.
- Covin RB, Evans KS, Olshock R, Thompson HW. Acute hemolytic transfusion reaction caused by anti-Coa. Immunohematology. 2001;17:45–49.
[Crossref] [Google Scholar] [PubMed]
- Krog GR, Rieneck K, Clausen FB, Steffensen R, Dziegiel MH. Blood group genotyping of blood donors: validation of a highly accurate routine method. Transfusion. 2019;59:3264–3274.
[Crossref] [Google Scholar] [PubMed]
- Bauer P, Kahwash E, Krugh D, Kennedy MS. A case of an antibody against a high frequency antigen. Lab Med. 2003;34:782–788.
- Marsh WL, Nichols ME, Oyen R, Thayer RS, Deere WL, Freed PJ, et al. Naturally occurring anti-Kell stimulated by E. coli enterocolitis in a 20-day-old child. Transfusion. 1978;18:149–154.
- Judd WJ, Walter WJ, Steiner EA. Clinical and laboratory findings on two patients with naturally occurring anti-Kell agglutinins. Transfusion. 1981;21:184–188.
[Crossref] [Google Scholar] [PubMed]
- Milkins C, Cantwell C, Elliott C, Haggas R, Jones J. Guidelines for pre-transfusion compatibility procedures in blood transfusion laboratories. Transfus Med. 2013;23:3–35.
[Crossref] [Google Scholar] [PubMed]
Citation: Kielsen K, Scharff BFSS, Hansen AT, Madsen LM, Clausen NE, Rieneck K, et al. (2023) Genotyping of Blood Donors for Timely Procurement of Rare Antigen-Negative Blood: A Case Story of Acute HTR and Anti-Coa. J Blood Disord Transfus. 14:553.
Copyright: © 2023 Kielsen K, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.