Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2019) Volume 8, Issue 3
Functional Neuroimaging Guided Active Rehabilitation for PCS: A Retrospective Comparative Study of an NVC-Targeting Therapeutic Approach
Caleb T Epps1, Marci L Johnson1,3, Alina K Fong1,2,4 and Mark D Allen1,2*2Notus Neuropsychological Imaging, Orem, Utah, USA
3Brigham Young University, Provo, Utah, USA
4Utah Valley Regional Medical Center, Provo, UT, USA
Received: 19-Oct-2019 Published: 05-Nov-2019
Abstract
Background: Recent work has demonstrated several advancements in therapeutic options for patients with postconcussion syndrome (PCS). Specifically, active rehabilitation has emerged as a promising direction for the best treatment outcomes. Enhanced Performance in Cognition (EPIC) is one form of active rehabilitation for PCS that uses a multi-disciplinary clinical approach focusing on two primary aims: the objective diagnosis of PCS using a quantitative, biomarker-based form of fMRI, and targeted neurorehabilitation. The targeting and rehabilitation of the neurovascular coupling (NVC) unit is an essential and novel component of this approach. This study seeks to contribute to the current field of active PCS therapy by demonstrating the usefulness of targeting NVC dysfunction. Further, we compare the EPIC protocol to treatment as usual using a retrospective comparative study design.
Methods: The principal cohort was designated as all patients who received EPIC treatment from the time original pilot data was published to the time of retrospective data analysis and chart review (June 2016-October 2017) (N=375). Pre- and post-EPIC post-concussion symptom scales (PCSS) and severity index scores (SIS) were measured and compared. Then, based on pre-specified inclusion criteria for chart review, two patient cohorts were retrospectively assigned to treatment order 1 (TO1: N=15) and treatment order 2 (TO2: N=28). SIS was measured at pre-/post-EPIC and pre-/post-treatment as usual (TAU) functional neurocognitive imaging scans. SIS results from the treatment periods were compared and reported.
Results: For the principal cohort (N=375), there was a statistically significant reduction in post-EPIC SIS and PCSS. Concerning the treatment cohorts, there was a highly significant (p<0.0001) overall effect of treatment type on SIS but no such effect of treatment order. However, analysis of TO2 alone revealed a significant effect on SIS reduction (p<0.001) if TAU occurs after EPIC therapy.
Conclusion: The findings in this study appear to agree with the current body of research that demonstrates the effectiveness of active rehabilitation strategies compared to standard symptom-modulating and rest-based PCS therapies. Further, EPIC-style therapies contribute to the field of active PCS treatment by addressing NVC disruption in addition to the autoregulatory/vasoreactive aspects of PCS pathophysiology.
Keywords
Post-concussion syndrome; Functional MRI; Neurocognitive rehabilitation; PCS rehabilitation; Mild traumatic brain injury; Neurovascular coupling
Abbreviations
PCS: Post-Concussion Syndrome; EPIC: Enhanced Performance in Cognition; SIS: Severity Index Score; PCSS: Post Concussion Symptom Scale; 1-TO1: Treatment Order Cohort; 2-TO2: Treatment Order Cohort; fNCI: Functional Neurocognitive Imaging; NVC: Neurovascular Coupling; TAU: Treatment as Usual
Key Points
• Data presented in the study supports the current trend of active PCS therapy by demonstrating objective and subjective improvement in PCS patients during periods of active treatment compared to treatment as usual.
• The study contributes to the field of active PCS management by demonstrating the possibility of NVC rehabilitation in addition to the autoregulatory/vasoreactive aspects of PCS pathophysiology.
• The study demonstrates the use of a novel fMRI protocol (fNCI) that is used to target areas of dysfunctional NVC in PCS patients.
Introduction
Treatment of post-concussion syndrome (PCS) is an actively evolving field with many recent developments in novel neurotherapeutic interventions from both practice-based and evidence-based research [1]. The current prevailing system of clinical management revolves around symptom reduction, which includes the avoidance of symptom provoking activities (rest) and pharmacologic intervention [2-6]. Other symptom-reduction strategies include vestibular physical therapy, cognitive rehabilitation, cognitive behavioural therapy, psychotherapy, physiotherapy, speech/language therapy, mindfulness, and meditation [7].
Although a strategy of rest and medication might reduce, or circumvent, some PCS symptoms, it largely fails to address the underlying chronic post-concussive pathophysiology, resulting in persistent impairments in cognitive, emotional, neuromuscular, cardiovascular, cerebrovascular, and autonomic functioning [8- 10]. There is a growing shift toward active rehabilitation methods for persistent PCS [11-14]. In particular, approaches that include aerobic exercise alone [15-18] and in conjunction with other neurorehabilitation therapies have shown promise [19]. A specific form of active rehabilitation within this class is termed Enhanced Performance in Cognition (EPIC) treatment. EPIC treatment is a multi-disciplinary approach to PCS clinical management that focuses on two primary aims: the objective diagnosis of PCS using a quantitative, biomarker-based form of fMRI and second, targeted neurorehabilitation of dysfunctional neural regions.
Among recent studies that promote exercise-centered (active), intervention there is an emerging opinion in which a hypothetical distinction is made between PCS patients who are deemed to have true physiological concussion, versus those whose symptoms are hypothesized to originate from sources other than primary metabolic/autonomic disturbances of concussion. It is further proposed that those with true physiological concussion may be identified on the basis of showing exercise induced symptom exacerbation, and that the diagnosis is confirmed by their subsequent responsiveness to exercise rehabilitation-such that when symptom exacerbation is eliminated, any remaining symptoms are assumed to no longer have a neuronal, metabolic, autonomic, vascular, or otherwise “physiological concussion” symptom generator [15]. Furthermore, because symptom response to physical exertion is taken as the primary indicator of physiological concussion, the primary mechanisms of disruption are assumed to be dysfunction of cerebral autoregulation (mediated by autonomic dysfunction), cerebrovascular reactivity, and/or low CO2 sensitivity [20]. On theoretical grounds, we do not fully agree with the opinion that autoregulatory mechanisms are the sole generators of ‘true’ PCS, nor do we agree that aerobic exercise-based rehabilitation is relevant only for those patients with exercise-induced symptom exacerbation. Instead, we have previously proposed [21] that disruption in the neurovascular coupling (NVC) system is an additional mechanism of PCS that has been shown to operate independently of cerebral autoregulation and which is also “truly physiological” in the sense of having a neuronal/glial/metabolic source. NVC is the proposed pro-active mechanism by which a neuron supplies itself with oxygenated blood at the initiation of a threshold level of activity [22]. A disruption to this complex but reliable signaling system, via mild traumatic injury, may result in physical and cognitive post-concussive symptoms, in the absence of exercise-induced symptom exacerbation-independent of autoregulatory/vasoreactive failures.
We have previously hypothesized that aerobic exercise challenge should become a core feature within in a PCS rehabilitation protocol, even for those who don’t display exercise symptom exacerbation, given its proposed role in restoring NVC signaling and other metabolically intensive neuronal operations. Briefly, the post-exercise cognitive boost provides a window in which neuronal functions can be “pushed” in rehabilitative activities that would otherwise be unsustainable for PCS patients with NVC dysfunction. Because fMRI is essentially a measure of NVC reliability [23,24], this method becomes relevant to assessment and treatment monitoring within an active rehabilitation protocol, such as EPIC, which is designed to address NVC dysfunction. Descriptions of the imaging method used in EPIC treatment, specific protocol details, as well as descriptions of the underlying pathophysiology that this approach entails, are found in previous reports [21,25- 27]. Previous publications are intended to provide enough detail that this method, or parts of it, could be incorporated into existing treatment programs.
An initial report of outcomes data from EPIC treatment in PCS patients was previously given in Wing et al. [26]. They measured the severity index score (SIS; See “Methods: Development of the severity index score” for further description) and the post-concussion symptom scale (PCSS) in 270 PCS patients pre- and post-EPIC treatment. They found apparent improvement in both SIS and PCSS in greater than 90% of PCS patients. This pilot data suggests that the EPIC protocol (or similar protocols) may serve as an effective approach within the active treatment class for PCS patients. Furthermore, this approach is rooted in an explicit theoretical framework [21]. A key limitation of the Wing et al. study was that they lacked a control group to which treatment was compared.
The present study compares EPIC therapy to standard practices, or “treatment as usual” (TAU) through a retrospective comparative analysis of patients who underwent both treatments. By so doing, we hope to contribute to the current field of active PCS therapy by demonstrating the usefulness of targeting NVC dysfunction. First, we aim to replicate and broaden the findings of previous pilot reports. Second, we present data from PCS patients who experienced both EPIC and TAU treatment in alternating order.
Methods
Overview
An overview of the methods used in this study may be found in Figure 1. The development of the functional neurocognitive imaging (fNCI) protocol, which serves as the foundation of EPIC treatment, and the subsequent normative atlas formation and PCS biomarker discovery/validation will be briefly summarized from previous reports. As shown in Figure 1, the principal cohort was designated as all patients who received EPIC treatment since the time the original pilot data [26] was published (N=375). Pre- and post-EPIC PCSS and SIS were measured and compared. Then, two patient cohorts were retrospectively assigned to treatment order 1 (TO1: N=15) and treatment order 2 (TO2: N=28) based on pre-specified inclusion criteria. The inclusion criteria for TO1 and TO2 will be discussed further in Section 2: Treatment Comparison. SIS was measured at pre-/post-EPIC and pre-/post- TAU fNCI scans. SIS results from the treatment periods were compared and reported.
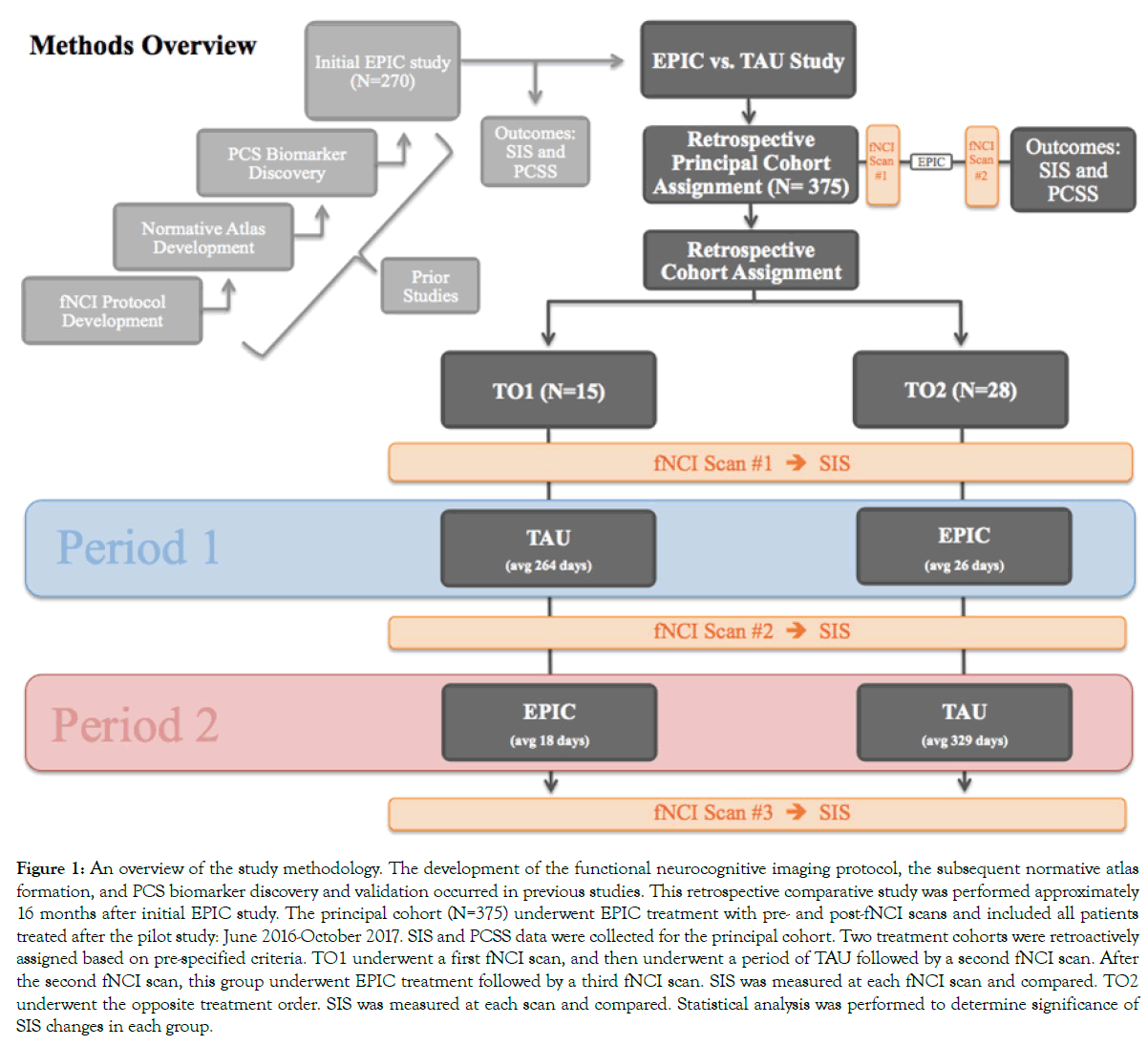
Figure 1: An overview of the study methodology. The development of the functional neurocognitive imaging protocol, the subsequent normative atlas formation, and PCS biomarker discovery and validation occurred in previous studies. This retrospective comparative study was performed approximately 16 months after initial EPIC study. The principal cohort (N=375) underwent EPIC treatment with pre- and post-fNCI scans and included all patients treated after the pilot study: June 2016-October 2017. SIS and PCSS data were collected for the principal cohort. Two treatment cohorts were retroactively assigned based on pre-specified criteria. TO1 underwent a first fNCI scan, and then underwent a period of TAU followed by a second fNCI scan. After the second fNCI scan, this group underwent EPIC treatment followed by a third fNCI scan. SIS was measured at each fNCI scan and compared. TO2 underwent the opposite treatment order. SIS was measured at each scan and compared. Statistical analysis was performed to determine significance of SIS changes in each group.
Development of the fNCI protocol, Normative Atlas, and Objective PCS Biomarkers
In order to meet the two primary aims of EPIC treatment, the objective diagnosis of PCS using a quantitative, biomarker-based form of fMRI and targeted neurorehabilitation, a valid fNCI protocol was developed. From the developed fNCI protocol, a normative atlas was formed from healthy volunteers and objective biomarkers were discovered and validated in PCS patients. Both of these steps occurred in prior studies but will be briefly reviewed below [27,28].
The fNCI assessment protocol combines the validity of conventional neuropsychological testing standards with the reliability and objectivity of informational data output provided by fMRI. The Notus NeuroCogs functional task battery employed in fNCI underwent iterative pilot testing to ensure concurrent validity, reliability, objectivity, and suitability for the MRI scanning environment [29-34], and is comprised of six neuropsychologic test adaptations: the functional Matrix Reasoning Test (f-MRT), the functional Trail Making Test-B (f-TMT), the functional Picture Naming Test (f-PNT), the functional Face Memory Test (f-FMT), the functional Verbal Memory Test (f-VMT), and the functional Verbal Fluency Test (f-VFT). During the fMRI, each patient will go through the battery of six cognitive tests. Patients complete some tasks with a button box and others via non-verbalized thought processes. Each of the six tasks includes eight test phases presented in alternating fashion with rest phases, in which the subject is asked to silently count from 1 to 10. Compliance monitoring is performed at intervals during each task. The fNCI protocol lasts approximately 45 minutes. Operative descriptions of each test were outlined in Epps et al. and Wing et al. [35]. Please refer to Wing et al. [35], section 2.1.2 “Standardized Protocol: Imaging Parameters”, for the specific imaging parameters used in the fNCI protocol.
In addition to the methods described here and elsewhere [29-35], anatomical regional normalization processes were applied, similar to Voyvodic [36], in order to improve inter-subject reliability and cross platform stability. This process is particularly relevant to the normative population, as data for these healthy controls were collected from four different scanning centers.
Once the fNCI protocol was developed, a normative atlas was formed using healthy volunteers. Data analysis revealed 57 specific functional regions (FR) found to be task-associated with each fNCI exam (8-12 FR’s per exam). A description of the normative atlas development can also be found in Epps et al. [28]. These functional regions were found to possess a normal distribution of activation patterns amongst reference subjects from which a three-dimensional activation standard or normative atlas was formulated. This was later used to statistically contextualize both severity and localization of the individual PCS patient activation patterns. Also, for an example of the assessment of activation patterns in individual patients compared to the normative atlas in all six fNCI exams [28] (Figures 2 and 3).
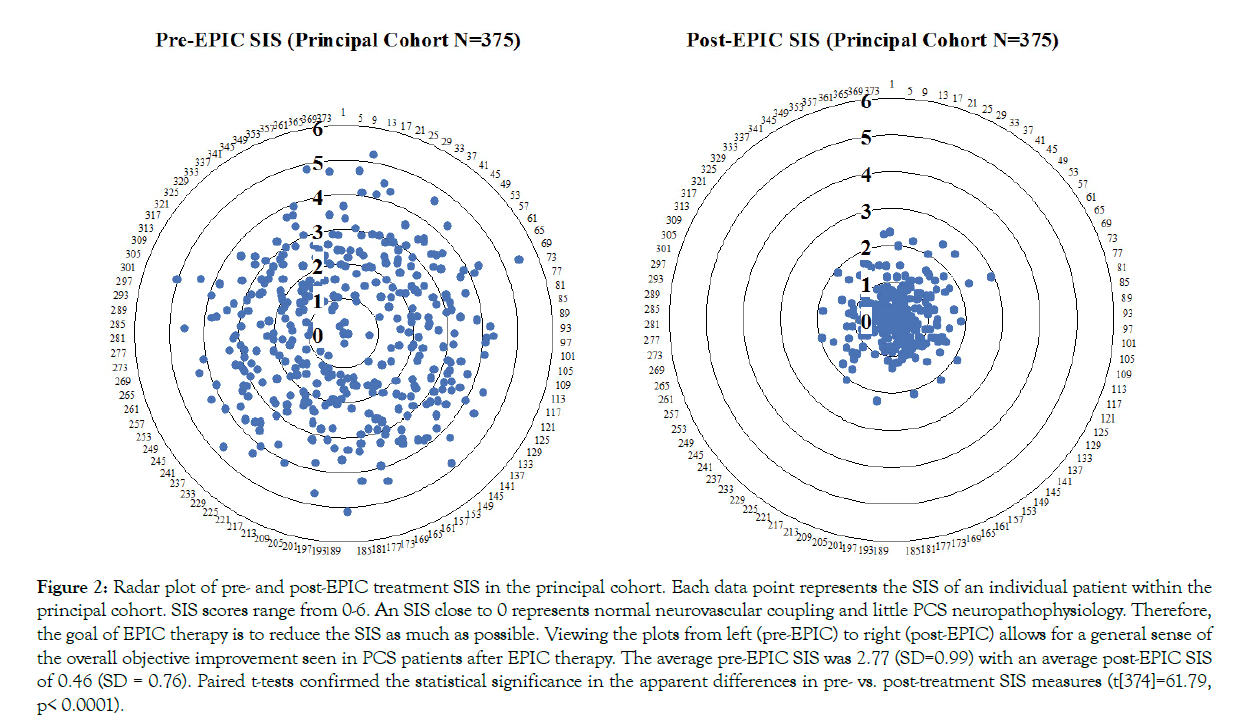
Figure 2: Radar plot of pre- and post-EPIC treatment SIS in the principal cohort. Each data point represents the SIS of an individual patient within the principal cohort. SIS scores range from 0-6. An SIS close to 0 represents normal neurovascular coupling and little PCS neuropathophysiology. Therefore, the goal of EPIC therapy is to reduce the SIS as much as possible. Viewing the plots from left (pre-EPIC) to right (post-EPIC) allows for a general sense of the overall objective improvement seen in PCS patients after EPIC therapy. The average pre-EPIC SIS was 2.77 (SD=0.99) with an average post-EPIC SIS of 0.46 (SD = 0.76). Paired t-tests confirmed the statistical significance in the apparent differences in pre- vs. post-treatment SIS measures (t[374]=61.79, p< 0.0001).
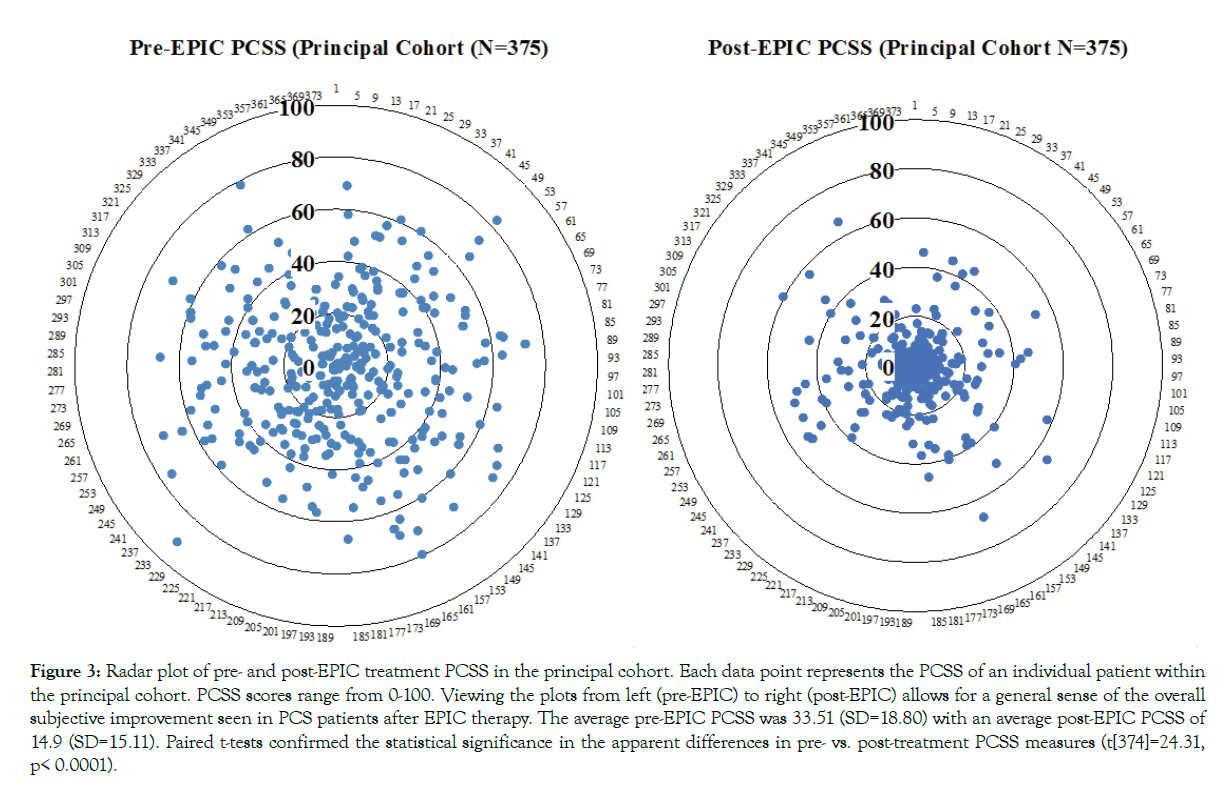
Figure 3: Radar plot of pre- and post-EPIC treatment PCSS in the principal cohort. Each data point represents the PCSS of an individual patient within the principal cohort. PCSS scores range from 0-100. Viewing the plots from left (pre-EPIC) to right (post-EPIC) allows for a general sense of the overall subjective improvement seen in PCS patients after EPIC therapy. The average pre-EPIC PCSS was 33.51 (SD=18.80) with an average post-EPIC PCSS of 14.9 (SD=15.11). Paired t-tests confirmed the statistical significance in the apparent differences in pre- vs. post-treatment PCSS measures (t[374]=24.31, p< 0.0001).
The normative reference atlas makes it possible to search for and verify biomarkers for specific pathologies, that is, reliable patterns of deviation from the norm associated with a specific pathology. Our PCS biomarker development followed a 3-step process: biomarker candidate search, independent samples validation, and multivariate base rate discovery. Using 259 PCS patients, Epps et al. [25] report the discovery of 5 PCS biomarkers in the Frontal Attentional System, Subcortical System, Visual System, Verbal System, and Frontal/Parietal System with sensitivities of 88%, 88%, 79%, 65%, and 41% respectively and specificities of 100%, 100%, 100%, 96%, 99% respectively. Further, they reported PCS diagnostic sensitivity of 88% and specificity of 99% if 3 of 5 biomarkers are below the 10th percentile.
Capitalize Severity Index Score
The fNCI SIS was developed to represent the overall presence of PCS biomarkers in an individual with a single summary score. The score is computed by taking the average activation deviation (z-score) across all target regions associated with a given biomarker within an individual and multiplying it by the positive predictive value for that biomarker. The SIS, then is the sum of this computation for all 5 biomarkers. For all PCS patients reported in Wing et al. [26], the SIS was found to have a mean of 5.11 (SD= 0.89), with an approximate range of 3-8. All healthy control subjects reported in Wing et al. [26] were similarly assessed for biomarker severity using the SIS scale, showing an average score of 2.01 (SD = 0.75) and approximate range of 1-4 (scores below 1 are nearly impossible with realistic brain activation variability). As the SIS is intended to be used primarily with patients who are independently diagnosed with probable PCS, as opposed to healthy controls, a patient-based SIS scale was developed in which the value 0 was set to the healthy control mean, such that scores tend to fall within the range 0-6. Simply put, as the quality of neurovascular coupling decreases within a given neuronal region, the SIS will increase in value.
EPIC treatment description
EPIC treatment and related imaging components have previously been reported in Wing et al. [35] and Epps et al. [25,21,28]. Using fNCI techniques, areas of deficient NVC are identified and targeted for rehabilitation. EPIC treatment integrates three fundamental neurocognitive rehabilitation components: Prepare, Activate, and Rest. Therapeutic activities used in each of these three phases are the result of research, clinical experience, screening, and empirical testing [37-41]. The preparatory stage includes aerobic exercise and neuromuscular therapy, titrated to patients exercise tolerance and lasts 50 minutes. The preparatory phase is designed to target dysregulations in the autoregulatory/vasoreactive systems, while preparing the brain for rehabilitation of areas with malfunctioning NVC. Aerobic challenge follows a sub-symptom threshold protocol for patients with residual exercise-induced symptom exacerbation. Following the preparatory stage is the activation stage, which includes 50 minutes of “complex multistep problem solving, logic puzzles, functional and short-term memory challenges, digital therapeutic games, visual exercises, motor skill retraining, and psychosocial therapy”, all of which is tailored to the neural regions that exhibited NVC deficits on fNCI [35]. If any visual spatial deficits were identified in the pre-treatment scan, visual spatial and sensorimotor therapeutic programs, including DynavisionTM and other commercial and in-house technologies are incorporated. The activation stage is followed by the rest phase, which includes an auditory binaural beats brainwave entrainment program to reduce stress while concurrently promoting cortico-thalamic synchrony of post-synaptic activity at frequency ranges that are often disrupted in PCS. The Preparation, Activation, and Rest phases are then repeated in cyclical fashion for 6-8 hours per day for 4 contiguous days and can be fine-tuned based upon symptom severity. It is important to note that the timing and order of these interventions are just as critical to successful treatment as are the interventions themselves. That is, careful and deliberate rotation between the aerobic challenge (preparation phase) and the cognitive challenge (activation phase) is a fundamental aspect in pairing regional cerebral blood flow and neuronal firing. The precision of this pairing requires a multidisciplinary team including athletic trainers, neuromuscular therapists, neurocognitive therapists, neurological occupational therapists, and clinical neuropsychologists. Lastly, post-EPIC fNCI is performed to identify the degree of normalization of NVC. Further treatment targeting neural areas of persistent NVC dysregulation may be performed.
Patient demographics
Patient data were collected from a single concussion management clinic specifically designed to implement EPIC therapy. Demographic information was collected via survey before patient began therapy. The clinical definition of PCS used for patient inclusion was a history of head trauma and associated onset of symptoms with persistence of those symptoms for greater than 3 months. (Table 1) displays demographic information for the principal cohort of 375 PCS patients, and breakout demographics for the two TO subgroups.
| Variables | Principal Cohort N=375 (%) | TO1 N=15 (%) | TO2 N=28 (%) | *p-value | |
|---|---|---|---|---|---|
| Sex | F | 193 (51) | 7 (47) | 13 (46) | 0.98 |
| M | 182 (49) | 8 (53) | 15 (54) | - | |
| Ethnicity | Caucasian | 334 (89) | 13 (87) | 27 (96) | 0.34 |
| Asian | 12 (3) | 1 (7) | 0 (0) | - | |
| Native-American | 4 (1) | 0 (0) | 0 (0) | - | |
| African-American | 4 (1) | 0 (0) | 0 (0) | - | |
| Hispanic | 20 (5) | 1 (7) | 1 (4) | - | |
| Other/No Response | 109 (29) | 0 (0) | 0 (0) | - | |
| Age | < 18 | 59 (16) | 1 (7) | 4 (14) | 0.7 |
| 18-64 | 298 (79) | 13 (87) | 23 (82) | - | |
| 65+ | 18 (5) | 1 (7) | 1 (4) | - | |
| Handedness | Right | 336 (90) | 15 (100) | 25 (89) | 0.42 |
| Left | 34 (9) | 0 (0) | 2 (7) | - | |
| Ambidextrous | 5 (1) | 0 (0) | 1 (4) | - | |
| Time Since Injury*** (Months) | < 12 | 168 (45) | 7 (47) | 14 (50) | 0.96 |
| 12-24 | 67 (18) | 6 (40) | 10 (36) | - | |
| 24-60 | 78 (21) | 2 (13) | 4 (14) | - | |
| 60+ | 62 (17) | 0 (0) | 0 (0) | - | |
| Injury Type | Sports-related | 111 (30) | 5 (33) | 14 (50) | 0.64 |
| Vehicular Accident | 149 (40) | 7 (47) | 10 (36) | - | |
| Pedestrian | 11 (3) | 0 (0) | 0 (0) | - | |
| Fall | 41 (11) | 1 (7) | 0 (0) | - | |
| Other | 63 (17) | 1 (7) | 4 (14) | - | |
| LOC/PTA | LOC & PTA | 79 (21) | 0 (0) | 0 (0) | 0.98 |
| LOC | 62 (17) | 4 (27) | 6 (21) | - | |
| PTA | 47 (13) | 1 (7) | 2 (7) | - | |
| Neither | 187 (50) | 12 (80) | 20 (71) | - | |
| Number of Concussions | 1 | 185 (49) | 9 (60) | 14 (50) | 0.81 |
| 02-04 | 142 (38) | 5 (33) | 12 (43) | - | |
| 05-07 | 37 (10) | 1 (7) | 1 (4) | - | |
| 8+ | 11 (3) | 0 (0) | 1 (4) | - | |
| Psychiatric History | Depression | 37 (10) | 3 (20) | 5 (18) | 0.99 |
| Anxiety | 25 (7) | 2 (13) | 3 (11) | - | |
| Depression & Anxiety | 67 (18) | 2 (13) | 4 (14) | - | |
| Other** | 46 (12) | 0 (0) | 2 (7) | - | |
| None | 225 (60) | 8 (53) | 14 (50) | - | |
Table 1: Patient demographics. Patients data was included in the study based on pre-specified inclusion criteria: A total of 418 patients were included (June 2016 -October 2017). All PCS patients were recruited from a single concussion treatment clinic. The clinical definition of PCS used for patient inclusion was a history of head trauma and associated onset of symptoms with persistence of those symptoms for greater than 3 months. Two treatment cohorts were retrospectively assigned to either TO1 or TO2 depending on defined criteria. *χ2 test TO1 vs. TO2. **Other Psychiatric History includes: ADD, ADHD, Bipolar, Schizophrenia, Dementia, PTSD, and ODD. *** Indicates time from injury to time of S1.
Section 1: Replication
Refer to Figure 1 for an overview of both sections of the study. For study section 1, a replication cohort was selected. All patients who underwent EPIC treatment from the end of the original pilot study [26] up to the time of data analysis for the current study (N=375; between June 2016 and October 2017) were included in the principal cohort (Table 1) for patient characteristics). This principal cohort underwent an initial fNCI (S1), followed by EPIC treatment that was tailored to each patient’s specific PCS biomarker abnormality profile [25]. After treatment, the patients underwent a second fNCI scan (S2). SIS was computed for both S1 and S2 scans. Data from the PCSS survey were collected prior to each scan and every day throughout their EPIC treatment.
Section 2: Treatment comparison
Section 2 of the study examined two cohorts (a total of 43 patients) who received both EPIC and TAU. A within subjects repeated-measures factor for the two treatment types (EPIC vs. TAU) and a between subjects counterbalanced factor for treatment order (Order 1=TAU-EPIC, Order 2=EPIC-TAU) analysis was performed.
TO1: As patients were undergoing treatment, it was noted that some declined to undergo EPIC treatment directly after S1. Further, many of these patients stated that they would consider undergoing treatment at a later date. Reasons for declining initial treatment included out-of-pocket expenses, denial of a longterm condition, and perceived complexity and duration of EPIC treatment regimen. Per the clinic’s practice standards, patients that fell into this category were instructed to continue standard PCS therapy as managed by their primary care provider and were not contacted further by clinical or research staff until they voluntarily contacted the clinic expressing intention to resume the assessment and treatment process. At the time of data analysis, a retrospective chart review discovered that 15 patients resumed the treatment process within the 16-month time-span since the principal cohort began treatment. This cohort was retrospectively designated as the TO1 group. All returning patients were determined to have engaged in treatment as usual, based on the criteria that they had all had follow-up contact with their primary care provider and engaged in any of the following interventions: medications (NSAID’s, anti-depressants, etc.), vestibular therapy, cognitive rehabilitation, cognitive behavioural therapy, psychotherapy, physiotherapy, speech and language therapy, and mindfulness and meditation. The TO1 cohort received a second fNCI scan upon deciding to resume assessment for EPIC treatment and begin the therapy, and a third fNCI scan upon completion of EPIC treatment. SIS was computed at S1, S2, and S3. The mean time between S1 and S2 (TAU interval) in this cohort was 264 days. The mean time between S2 and S3 (EPIC interval) was 18 days.
TO2: At approximately one year from the end of the pilot study cohort [26], and the beginning of what would be the principal cohort of this study, a random group of patients were selected to participate in a third-follow-up-fNCI scan (S3) to assess the long-term stability of their PCS biomarkers. Random selection occurred by assigning each member of the principal cohort a random number via a random number generator. Patients were then contacted in numerical order based on their assigned number. Selection was limited to only those for whom at least 9 months had elapsed since the end of EPIC treatment. Selection continued, with a rolling 3-month eligibility window until the time the retrospective data analysis occurred. Twenty-eight participants returned for S3 during this time period (July-October 2017). This cohort was retrospectively designated as the TO2 group. The TO2 cohort had received previous fNCI scans pre- and post-EPIC treatment (S1 and S2). SIS computed at S1, S2, and S3 were compared. Further, per chart review, it was found that all twenty-eight patients had participated in continued symptom reduction activity in between S2 and S3 as guided by their primary providers. The mean time between S1 and S2 in this cohort was 26 days. The mean time between S2 and S3 was 329 days. It is important to note that although the TO2 cohort was selected from the principal cohort, their data is separate from and was not included in the principal cohort’s SIS and PCSS data.
One detail concerning scan intervals for these two groups should be noted. Although EPIC treatment lasts 4-5 days, mean scan intervals for the EPIC periods were approximately 20 days. This is because some patients received the first scan 1-2 weeks before EPIC treatment in order to better accommodate the patients’ individual scheduling needs. However, the second scan was always performed immediately following EPIC treatment. All patients underwent additional structural MRI brain scanning at the time of their first fNCI scan. Patients with positive findings were not included in the study.
Results
Section 1: Replication
For the principal cohort of 375 PCS patients, the average pre-EPIC SIS was 2.77 (SD=0.99) with an average post-EPIC SIS of 0.46 (SD=0.76). The average pre-EPIC PCSS was 33.51 (SD=18.80) with an average post-EPIC PCSS of 14.9 (SD=15.11). A summary of pre- and post-EPIC SIS and PCSS may be found in Figures 2 and 3. Paired t-tests confirmed the apparent differences in pre- vs. post-treatment SIS measures (t[374]=61.79, p<0.0001) and PCSS measures (t[374]=24.31, p<0.0001).
Section 2: Treatment comparison
TO1: SIS outcomes for patients in the TO cohorts are as follows: for the TO1 cohort of 15 PCS patients, the average initial fNCI (S1) SIS was 3.1 (SD=0.8). The average SIS from the post-treatment scan following TAU was 3.0 (SD=0.6). The average SIS from the post-treatment scan following EPIC was 0.3 (SD=0.6). The mean time between S1 and S2 (TAU period) in this cohort was 264 days. The mean time between S2 and S3 (EPIC period) was 18 days. A summary of the results for the TO1 cohort is shown in Figure 4.
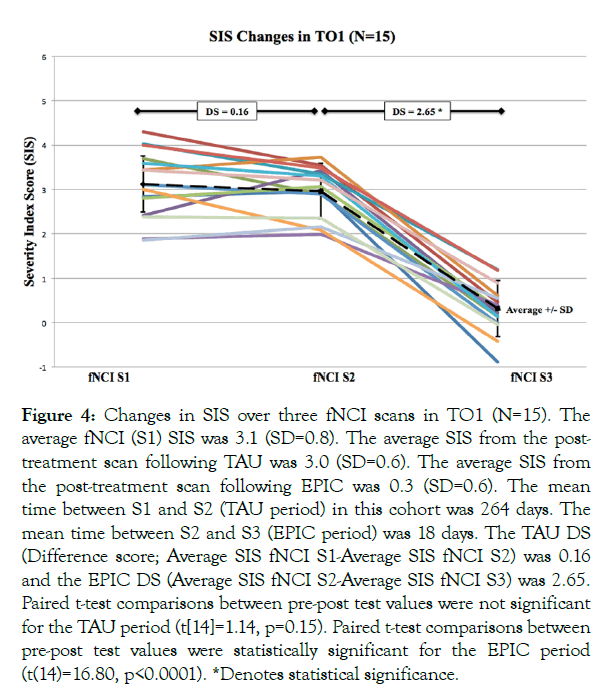
Figure 4: Changes in SIS over three fNCI scans in TO1 (N=15). The average fNCI (S1) SIS was 3.1 (SD=0.8). The average SIS from the post-treatment scan following TAU was 3.0 (SD=0.6). The average SIS from the post-treatment scan following EPIC was 0.3 (SD=0.6). The mean time between S1 and S2 (TAU period) in this cohort was 264 days. The mean time between S2 and S3 (EPIC period) was 18 days. The TAU DS (Difference score; Average SIS fNCI S1-Average SIS fNCI S2) was 0.16 and the EPIC DS (Average SIS fNCI S2-Average SIS fNCI S3) was 2.65. Paired t-test comparisons between pre-post test values were not significant for the TAU period (t[14]=1.14, p=0.15). Paired t-test comparisons between pre-post test values were statistically significant for the EPIC period (t(14)=16.80, p<0.0001). *Denotes statistical significance.
TO2: For the TO2 cohort of 28 patients, the average initial fNCI (S1) was 3.3 (SD=0.7). The average SIS from the post-treatment scan following EPIC was 0.8 (SD=0.9), and the average SIS from the post-treatment scan following TAU was 0.3 (SD=0.5). The mean time between S1 and S2 in this cohort was 26 days. The mean time between S2 and S3 was 329 days. A summary of the results for the TO2 cohort is shown in Figure 5.
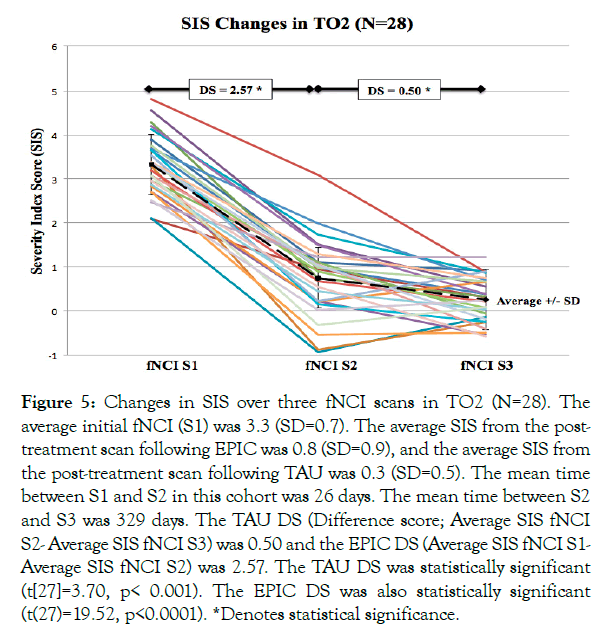
Figure 5: Changes in SIS over three fNCI scans in TO2 (N=28). The average initial fNCI (S1) was 3.3 (SD=0.7). The average SIS from the post-treatment scan following EPIC was 0.8 (SD=0.9), and the average SIS from the post-treatment scan following TAU was 0.3 (SD=0.5). The mean time between S1 and S2 in this cohort was 26 days. The mean time between S2 and S3 was 329 days. The TAU DS (Difference score; Average SIS fNCI S2- Average SIS fNCI S3) was 0.50 and the EPIC DS (Average SIS fNCI S1- Average SIS fNCI S2) was 2.57. The TAU DS was statistically significant (t[27]=3.70, p< 0.001). The EPIC DS was also statistically significant (t(27)=19.52, p<0.0001). *Denotes statistical significance.
Section 3: Statistical analyses
For each patient in both TO groups, an SIS difference score (pre-vs. post-treatment) was calculated for each treatment type. For TO1, the TAU difference score was the change from S1 to S2 and the EPIC difference score was the change from S2 to S3. For the TO2 group, the TAU difference score was the change from S2 to S3 and the EPIC difference score was the change from S1 to S2. The mean SIS difference scores were as follows: TO1 TAU difference=0.16, EPIC difference=2.65; TO2 TAU difference=0.50, EPIC difference=2.57. Pooling individual difference scores across both TO groups, mean difference scores were TAU=0.38 and EPIC=2.60.
Differences among change scores were analysed using a mixed-model rANOVA with Treatment Type (TAU vs. EPIC) as a within-subject variable and Order (Order 1 vs. Order 2) as a between-subject factor. The main effect for Treatment Type was highly significant (F[1,41]=161.33, p<0.0001, confirming the large numerical difference between treatment difference means. The main effect for Order was not significant (F[1,41]=1.47, p=0.23). The interaction between Treatment Type and Order was not significant (F[1,41]=1.33, p=0.25). Although not significant at critical thresholds, the main effect for Order and the interaction effect showed hints toward significance. The source of this trend is apparent, given the observation of relatively greater improvement in TAU for TO2 compared with TO1 (Figures 4 and 5). Post-hoc pair-wise comparison analyses using paired t-tests for individual pre- vs. post-treatment SIS changes explored this further. For the TO2 group order (TAU second), the TAU change was statistically significant (t[27]=3.70, p<0.001), whereas for the TO1 group (TAU first), the TAU change was not significant (t[14]=1.14, p=0.15). For EPIC, paired t-tests for individual pre- vs. post-treatment SIS changes were statistically significant regardless of order (TO1 [t(14)=16.80, p<0.0001]; TO2 [t(27)=19.52, p<0.0001]).
Discussion
Within the emerging field of active PCS rehabilitation, the outcomes reported in Wing et al. [26] raised the possibility that approaches involving NVC rehabilitation, such as the EPIC protocol could be a reliable and effective intervention in PCS patients. In the first section of the current study, we replicated and broadened the main findings reported in Wing et al. [26]; reporting SIS and PCSS changes pre- and post-EPIC in a new sample of 375 PCS patients. In the second section, we presented the data of 43 PCS patients who underwent both EPIC and standard treatment protocols (TAU), in counterbalanced order. SIS from each treatment period in the two cohorts (TO1; TO2) pre- and post-treatment was calculated, compared, and statistical significance determined.
Considering the principal cohort first, (Figures 2 and 3) demonstrates overall improvement and statistically significant reductions in both SIS and PCSS post-EPIC treatment. This replicates the work reported by Wing et al. and broadens their findings by providing the statistical testing necessary to validate the changes observed.
A conceivable advantage of this approach is that it works within a theoretical model that makes clear assumptions about underlying chronic concussive pathophysiology-an approach that not only aims to improve ANS and cerebral autoregulatory function with structured aerobic exercise, but also to restore proper NVC function with adjunctive cognitive and sensorimotor therapies. These findings suggest that EPIC and EPIC-style treatments address regions of disrupted NVC (as evident by the fMRI biomarker-based SIS results) as well as global cerebral autoregulatory and vasoreactive dysfunction.
There was a marked decrease in mean SIS from S2 to S3 (EPIC) and a minimal decrease from S1 to S2 (TAU) in the TO1 cohort (Figure 4). Considering TO2, there was a marked decrease in mean SIS from S1 to S2 (EPIC) and a minimal decrease in mean SIS from S2 to S3 (TAU) (Figure 5). Of interest, the TAU difference score observed in TO2 (TAU second) was more than double the TAU difference score observed in TO1 (TAU first).
Differences among SIS were analysed for effects of Treatment Type (TAU vs. EPIC), Order of Treatment (Order 1 vs. Order 2), and interactions between treatment type and order. Considering Treatment Type as a main effect collapsed across order, patients treated with EPIC compared to TAU showed an overall statistically significant decrease in mean SIS. This provides further evidence that greater reductions in SIS, and therefore PCS rehabilitation secondary to NVC re-regulation, occur during the EPIC period compared to the TAU period. Considering Order of Treatment collapsed across treatment types, there was no statistically significant change in mean SIS. However, as mentioned previously, the mean TAU difference score in TO2 was more than double that of TO1 (0.50 and 0.16, respectively) (Figures 4 and 5). This apparent interactive effect prompted a post-hoc analysis of pre- versus post-treatment SIS scores within TO1 and TO2 cohorts independently for each treatment type. This analysis revealed a significant decrease in mean SIS during the TAU phase for the TO2 cohort (TAU second) but not for the TO1 cohort (TAU first). One explanation for these findings is that the NVC re-regulation that occurs during EPIC treatment continues to contribute to PCS rehabilitation even after active treatment has ceased. Of note, the decreases in mean SIS during EPIC for TO1 and TO2 were statistically significant regardless of treatment order. Therefore, there seems to be no beneficial effects from participating in TAU by itself prior to EPIC treatment, nor did having had TAU first appear to benefit the efficacy of subsequent EPIC treatment. Furthermore, patients participated in TAU over a significantly longer period of time than EPIC. This is a highly conservative element of the study, given that if time alone were a contributing factor to PCS recovery, TAU would have been given a substantial advantage over EPIC treatment. Thus at the very least, these results show that active rehabilitation proved more beneficial than what would be expected with symptom resolution over time alone, regardless of individual differences in TAU treatment variation among patients.
Active rehabilitation methods for persistent PCS have shown promising results [12-14]. Specifically, aerobic exercise has been documented to be an effective option in PCS management [15-18]. Aerobic exercise in conjunction with other neurorehabilitation therapies has also shown promise [11,19]. Of note, how these studies have shown improvement is largely based on the reduction of symptom exacerbation during exercise, using sub-symptom threshold protocols. Because of the success of this approach in reducing PCS symptoms that are most typically induced by physical exertion (i.e., headache, dizziness, and nausea), the primary mechanisms of pathology in PCS are assumed to include only those neurovascular systems that give rise to these symptoms when dysfunctional-namely cerebral autoregulation (mediated by autonomic dysfunction), cerebrovascular reactivity, and/or low CO2 sensitivity [24]. As previously mentioned however, another mechanism of PCS pathology- separate from autoregulatory/ vasoreactive failures-involves the disruption of the NVC mechanism. We argue that NVC signaling plays a considerable role in PCS pathology, given the strong record of consistent findings of disrupted focal activity in fMRI studies of mild TBI [42-44]. Also, NVC disruption would account for not only the physical symptoms observed in PCS (along with cerebral auto regulation mechanisms), but also the cognitive and sensorimotor deficits observed in chronic post-concussive symptoms. Moreover, NVC disruption would explain a host of chronic PCS symptoms beyond headache and dizziness, and further explain why these symptoms occur at times other than during physical exercise. Finally, therapies designed to restore NVC function prescribe aerobic exertion as a core therapy, beyond its use in sub-symptom threshold protocols. The data presented in this report suggest that EPIC, and other EPIC-style therapies contribute to the field of active PCS rehabilitation by addressing both autoregulatory/vasoreactive and NVC pathology that occur in PCS.
The current study included three limitations: the TAU treatment (control variable) was not randomly assigned or supervised as would be in traditional head-to-head treatment comparisons, lack of widely accepted use of SIS as a measure of PCS severity, and a lack of standard clinical data for the TO1 and TO2 cohorts (i.e., PCSS scores). Each of these limitations will be considered individually.
First, the two treatment cohorts were not randomized into their assigned groups and no oversight was given during the TAU period. As described in the methods, it is standard protocol of the concussion treatment clinic where patient data was analysed that any patient who a) declines EPIC therapy after initial scan or b) is treated and receives S2, is instructed to continue follow up with their primary provider to help address symptoms. Further, at the time of return, all 43 patients confirmed engaging in PCS symptom-reduction activity. It should also be reinforced that evidence concerning the benefits of non-pharmacological and pharmacological interventions targeting PCS is limited and conflicting [44]. As there is no “gold standard” to which PCS management stratagems may be compared to in head-to-head analyses, any efforts made at symptom reduction may be classified as “TAU”. Lastly, the three cohorts did not differ in any meaningful way demographically or with initial PCS severity (Table 1). The initial average SIS of fNCI S1 for the three cohorts did not differ in any significant way. This suggests that severity of PCS at time of treatment was similar for all groups. Further, the time spent in each treatment period for each cohort was similar.
Second, SIS has not gained widespread acceptance as a measure of PCS severity but serves as the primary outcome measure in this study. This is inherent with the advanced nature of the imaging. Further, there has been little demand for an objective measure of NVC dysfunction. Given the nature of the diagnostic and therapeutic methods of the EPIC protocol, SIS is the most applicable objective measure. As the role of functional neuroimaging becomes more and more clinically relevant, objective measures will become increasingly important. This study further demonstrates its relevance and provides more exposure. The measure was consistently applied amongst all three groups and there are no built-in biases in its calculation. Lastly, objective clinical data such as PCSS for the TO1 and TO2 cohorts are not reported. This is due to incomplete data gathered from these cohorts. Due to their small sample sizes, even the exclusion of a few patients PCSS scores obscures the data. However, because of the much larger sample size in the principal cohort, an accurate depiction of PCSS, and therefore clinical improvement, was possible.
Conclusion
In conclusion, the findings in this study appear to agree with the current body of research that demonstrates the effectiveness of active rehabilitation strategies compared to standard symptommodulating and rest-based PCS therapies. EPIC-style therapies contribute to the field of active PCS treatment by addressing NVC disruption in addition to the autoregulatory/vasoreactive aspects of PCS pathophysiology. These findings replicate and extend the results reported in Wing et al. who demonstrated improvement in SIS and PCSS post-EPIC therapy. This retrospective comparative study helps to address key limitations found in Wing et al. by providing a control to which the EPIC treatment may be compared. Other limitations of the Wing et al. study were addressed by providing statistical testing validating the improvements seen in SIS. It is important to note that the presented data only spans the course of 16 months. Future data will need to be analysed in order to investigate longer-term beneficial effects. However, key limitations to our study include the non-randomization of TO1, a lack of oversight during the TAU treatment period, small sample sizes, partially accepted use of SIS as a measure of PCS severity, and a lack of PCSS data in the TO1 and TO2 groups. Future studies may include a) prospective, randomized control trials of EPIC-style therapies, b) the use of a homogenous PCS patient populations (i.e., patients with sports-related concussion only), and c) the use of widely accepted clinical measures of patient improvement in the treatment and control groups.
Ethical Approval and Informed Consent
This analysis was approved by the institutional review board and ethics committee of Notus Imaging Research Laboratory (reference numbers 2016-03, 2017-03). It is the standard practice of the concussion treatment clinic where patient data was collected that patients provide written informed consent allowing their data to be used for research purposes. Each patient was informed as to the nature of participation in EPIC, risks, benefits, how their confidentiality would be protected, and contact information for any additional questions.
Consent for Publication
Not applicable
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This research did not receive any funding or support from agencies in the public, commercial, or not-for-profit sectors.
Competing Interest
All authors have financial involvement with Cognitive Fx, the private concussion treatment clinic where patients were treated and data gathered for the study. Drs. Fong and Allen are co-owners of Cognitive FX (Provo, UT, USA). Epps CT is an employed research associate of the clinic. Johnson ML is the director of treatment at Cognitive Fx.
Authors Contributions
CTE interpreted data, developed tables and figures, and drafted the manuscript. MLJ obtained data, developed and configured figures, and edited the manuscript. AKF contributed to manuscript editing and MDA was key in the development of the study protocol and editing of tables, figures, and manuscript. All authors read and approved the final manuscript.
Acknowledgement
Not applicable.
REFERENCES
- Hadanny A, Efrati S. Treatment of persistent post-concussion syndrome due to mild traumatic brain injury: current status and future directions. Expert Rev Neurother. 2016;16:875-887.
- Maroon JC, Lepere DB, Blaylock RL, Bost JW. Post-concussion syndrome: A review of pathophysiology and potential nonpharmacological approaches to treatment. Phys Sportsmed. 2012;40:73-87.
- Evans RW. Post-traumatic headaches. Neurol Clin. 2004;22:237-249.
- Mittenberg W, Canyock EM, Condit D, Patton C. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol. 2001;23:829-836.
- Fann JR, Bombardier CH, Temkin N, Esselman P, Warms C, Barber J, et al. Sertraline for major depression during the year following traumatic brain injury: A randomized controlled trial. J Head Trauma Rehabil. 2017;32:332-42.
- Fann JR, Uomoto JM, Katon WJ. Cognitive improvement with treatment of depression following mild traumatic brain injury. Psychosomatics. 2001;42:48-54.
- Alsalaheen BA, Whitney SL, Mucha A, Morris LO, Furman JM, Sparto PJ. Exercise prescription patterns in patients treated with vestibular rehabilitation after concussion. Physiother Res Int. 2013;18:100-108.
- Chapman EH, Weintraub RJ, Milburn MA, Pirozzi TO, Woo E. Homeopathic treatment of mild traumatic brain injury: A randomized, double-blind, placebo-controlled clinical trial. J Head Trauma Rehabil. 1999;14:521-542.
- McAllister TW, Arciniegas D. Evaluation and treatment of post-concussive symptoms. Neurorehabilitation. 2002;17:265-283.
- Origenes AK, Alleva JT, Hudgins TH. Concussion rehabilitation/post-concussion syndrome. Dis Mon. 2019.
- Sullivan KA, Hills AP, Iverson GL. Graded Combined Aerobic Resistance Exercise (CARE) to prevent or treat the persistent post-concussion syndrome. Curr Neurol Neurosci Rep. 2018;18:75.
- Leddy JJ, Baker JG, Willer B. Active rehabilitation of concussion and post-concussion syndrome. Phys Med Rehabil Clin N Am. 2016;27:437-47-54.
- McClincy MP, Lovell MR, Pardini J, Collins MW, Spore MK. Recovery from sports concussion in high school and collegiate athletes. Brain Inj. 2006;20:33-39.
- Leddy JJ, Haider MN, Ellis M, Willer BS. Exercise is medicine for concussion. Curr Sports Med Rep. 2018;17:262-70.
- Kurowski BG, Hugentobler J, Quatman-Yates C, Taylor J, Gubanich PJ, Altaye M, et al. Aerobic exercise for adolescents with prolonged symptoms after mild traumatic brain injury: An exploratory randomized clinical trial. J Head Trauma Rehabil. 2017;32:79-89.
- Chrisman SPD, Whitlock KB, Somers E, Burton MS, Herring SA, Rowhani-Rahbar A, et al. Pilot study of the Sub-Symptom Threshold Exercise Program (SSTEP) for persistent concussion symptoms in youth. Neurorehabilitation. 2017;40:493-499.
- Lal A, Kolakowsky-Hayner SA, Ghajar J, Balamane M. The effect of physical exercise after a concussion: A systematic review and meta-analysis. Am J Sports Med. 2018;46:743-752.
- Grabowski P, Wilson J, Walker A, Enz D, Wang S. Multimodal impairment-based physical therapy for the treatment of patients with post-concussion syndrome: A retrospective analysis on safety and feasibility. Phys Ther Sport. 2017;23:22-30.
- Clausen M, Pendergast DR, Willer B, Leddy J. Cerebral blood flow during treadmill exercise is a marker of physiological postconcussion syndrome in female athletes. J Head Trauma Rehabil. 2016;31:215-224.
- Epps C, Allen MD. Neurovascular coupling: A unifying theory for post-concussion syndrome treatment and functional neuroimaging. Journal of Neurology and Neurophysiology. 2017;8.
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232-243.
- Hillman EM. Coupling mechanism and significance of the BOLD signal: A status report. Annu Rev Neurosci. 2014;37:161-81.
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133-1145.
- Epps C, Allen MD. Discovery of therapy-targeting biomarkers for post-concussion syndrome using functional neurocognitive imaging. Brain Disorders and Therapy. 2018;7:1-2.
- Wing BH, Tucker BJ, Fong AK, Allen MD. Developing the standard of care for post-concussion treatment: neuroimaging-guided rehabilitation of neurovascular coupling. Open Neuroimag J. 2017;11:58-71.
- Allen MD WT, Bigler ED. Traumatic brain injury alters word memory test performance by slowing response time and increasing cortical activation: An fMRI study of a symptom validity test. Psychological Injury and Law. 2011;4:140-146.
- Epps CT TB, Fong AK, Allen MD. Functional neurocognitive imagingtm and notus neurocogsTM: A novel use of fMRI in the assessment of cognitive function in major neurocognitive disorder. J Neuroimaging Psychiatry Neurol. 2017;2:20-29.
- Allen MD, Fong AK. Clinical application of standardized cognitive assessment using fMRI. I. Matrix reasoning. Behav Neurol. 2008;20:127-140.
- Allen MD, Fong AK. Clinical application of standardized cognitive assessment using fMRI. II. Verbal fluency. Behav Neurol. 2008;20:141-152.
- Allen MD, Hedges DW, Farrer TJ, Larson MJ. Assessment of brain activity during memory encoding in a narcolepsy patient on and off modafinil using normative fMRI data. Neurocase. 2012;18:13-25.
- Allen MD, Owens TE, Fong AK, Richards DR. A functional neuroimaging analysis of the Trail Making Test-B: implications for clinical application. Behav Neurol. 2011;24:159-171.
- Woon FL, Allen MD, Miller CH, Hedges DW. The functional magnetic resonance imaging-based verbal fluency test in obsessive-compulsive disorder. Neurocase. 2012;18:424-440.
- Allen MD WT, Bigler ED. Traumatic brain injury alters word memory test performance by slowing response time and increasing cortical activation: An fMRI study of a symptom validity test. Psychol Inj Law. 2011;4:140-146.
- Wing BH TB, Fong AK, Allen MD. Developing the standard of care for post-concussion treatment: Neuroimaging-guided rehabilitation of neurovascular coupling. The Open Neuroimaging Journal. 2017(11):3-16.
- Voyvodic JT. Reproducibility of single-subject fMRI language mapping with AMPLE normalization. J Magn Reson Imaging. 2012;36:569-580.
- Duff J. The usefulness of quantitative EEG (QEEG) and neurotherapy in the assessment and treatment of post-concussion syndrome. Clin EEG Neurosci. 2004;35:198-209.
- Leddy J, Hinds A, Sirica D, Willer B. The role of controlled exercise in concussion management. PM R. 2016;8:91-100.
- Makdissi M, Schneider KJ, Feddermann-Demont N, Guskiewicz KM, Hinds S, Leddy JJ, et al. Approach to investigation and treatment of persistent symptoms following sport-related concussion: A systematic review. Br J Sports Med. 2017;51:958-968.
- Sawyer Q, Vesci B, McLeod TC. Physical activity and intermittent post-concussion symptoms after a period of symptom-limited physical and cognitive rest. J Athl Train. 2016;51:739-42.
- Schneider KJ, Leddy JJ, Guskiewicz KM, Seifert T, McCrea M, Silverberg ND, et al. Rest and treatment/rehabilitation following sport-related concussion: a systematic review. Br J Sports Med. 2017;51:930-934.
- Dona O, Noseworthy MD, DeMatteo C, Connolly JF. Fractal analysis of brain blood oxygenation level dependent (BOLD) signals from children with mild traumatic brain injury (mTBI). PLoS One. 2017;12.
- Bartnik-Olson BL, Holshouser B, Wang H, Grube M, Tong K, Wong V, et al. Impaired neurovascular unit function contributes to persistent symptoms after concussion: A pilot study. J Neurotrauma. 2014;31:1497-506.
- Mikulis DJ. Chronic neurovascular uncoupling syndrome. Stroke. 2013;44:55-57.
- Polinder S, Cnossen MC, Real RGL, Covic A, Gorbunova A, Voormolen DC, et al. A multidimensional approach to post-concussion symptoms in mild traumatic brain injury. Front Neurol. 2018;9:1113.
Citation: Epps CT, Allen MD, Johnson ML, Fong AK (2019) Functional Neuroimaging Guided Active Rehabilitation for PCS: A Retrospective Comparative Study of an NVC-targeting Therapeutic Approach. Brain Disord Ther 8:255. doi: 10.35248/2168-975X.19.8.255
Copyright: © 2019 Epps CT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


