Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- Google Scholar
- Quality Open Access Market
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 9, Issue 6
Efficacy of the ShotBlocker® for Pain Management During Needle-related Procedures: A Systematic Review and Meta-Analysis
Birsen Bilgen Sivri*, Marlene Berger and Cornelia MahlerReceived: 17-Sep-2023, Manuscript No. JPMME-23-23079; Editor assigned: 19-Sep-2023, Pre QC No. JPMME-23-23079 (PQ); Reviewed: 03-Oct-2023, QC No. JPMME-23-23079; Revised: 10-Oct-2023, Manuscript No. JPMME-23-23079 (R); Published: 20-Oct-2023
Abstract
Background: Children are exposed to various painful procedures (e.g., blood withdrawal, IM/Sc injection, vaccination, and IV catheterization). This systematic review and meta-analysis examined the effectiveness of the ShotBlocker® for needle-related procedural pain in children.
Materials and Methods: A literature search was conducted covering English, German, and Turkish articles studying 0-18 years-old individuals. Databases were searched from April to June 2021 to identify Randomized Controlled Trials (RCTs) using the ShotBlocker® for pain management in children undergoing needle-related procedures. Selection of studies, data extraction, and assessment of risk of bias and quality of evidence were independently performed by two reviewers.
Results:A total of 10 studies involving 1121 participants aged to 18 years were included in the systematic review, and 8 were suitable for meta-analysis. The meta-analysis compared the ShotBlocker®, cold and vibration device, Usual Care, and Placebo. The meta-analysis compared the ShotBlocker® device with a no-treatment comparator and the effect of the device was significant in reducing self-reported procedural pain (MD=−0.67, 95% CI: −0.84, −0.51, I2 =84%, P< 0.00001), observer-reported pain (MD=−0.75, 95% CI: −0.88, −0.61, I2 =89%, P< 0.00001), self-reported procedural fear/anxiety (MD=−1.12, 95% CI: −1.41, −0.84, I2 =95%, P< 0.00001), observer-reported fear/anxiety (MD=−1.18, 95% CI: −1.38, −0.97, I2 =92%, P<0.00001). But compared to cold and vibration, the effect of the device was not significant in reducing observer-reported procedural pain (MD=0.55, 95% CI: 0.33, 0.77, I2 =91%, P< 0.00001).
Conclusion: Although ShotBlocker® is effective in reducing pain and fear/anxiety in some studies and not effective in some others, it is non-invasive; there is no need to wait an effect, and a harmless device.
Keywords
ShotBlocker®; Pain management; Procedural pain; Needle-related procedures; Children
Introduction
Pain is defined by the International Pain Research Society Taxonomy Committee as an unpleasant emotional and biochemical state or behavior that originates from a certain part of the body, is due to tissue damage or not, and is affected by past experiences (IASP-https://www.iasp-pain.org/) [1]. Children are exposing to various needle-related procedures from the newborn period [2]. Painful procedures such as blood sample, injection, Intravenous (IV) catheter insertion, and vaccination in children are one of the biggest sources of fear, anxiety, needle phobia, and stress [3,4]. These fears often lead to reluctance in the child and his family towards medical procedures and affect the child's subsequent treatment and care experience [5-7]. Ensuring timely and effective pain management during painful procedures applied to the child will also increase the tolerance to pain in later applications [8].
To counter the issues noted, pain management has included pharmacological and non-pharmacological methods. Pharmacological methods, including topical anesthetics (e.g., EMLA®), are expensive and require a long wait for the analgesic effect to be felt [9]. Non-pharmacological methods include cognitive-behavioral techniques and peripheral-physical techniques (e.g., ShotBlocker®, Buzzy®). Non-pharmacological methods are noninvasive, inexpensive, have no side effects, and are independent functions of the health professional [3,8].
Cognitive-behavioral techniques act through changes in sensory factors in relieving pain, anxiety, and stress. Studies have shown that applications such as bubble blowing, therapeutic clowning, and kaleidoscope, watching cartoons, music, and using distraction cards reduce pain, and anxiety in children [10-14].
Peripheral techniques include massage, touching, rubbing, acupuncture, reflexology, hot/cold/menthol application to the skin, vibration, and Transcutaneous Electrical Nerve Stimulation (TENS) [15-17]. Melzack and wall, who developed the Gate Control Theory, reported that the presence and severity of pain depend on the transmission of neurological stimuli, and then the presence of cognitive-cognitive effects may also play a role in the modulation of pain [18]. One of the devices used together with the effect of cold and vibration is Buzzy® (MMJ Labs, Atlanta, GA, USA). It has been observed that Buzzy® reduces pain and anxiety in painful procedures, such as blood drawing, intramuscular injection, intravenous catheterization, vaccination, and insulin injection in children in different age groups [5,19-22]. In addition, 4 metaanalyses and systematic reviews recently examined the effectiveness of Buzzy® in painful procedures, and it has been concluded that it reduces pain [20,23-25]. However, Buzzy® is very expensive at $60 per piece and it is necessary to wait at least 10 minutes in the refrigerator for the gel-filled package placed behind Buzzy® to freeze (https://paincarelabs.com/).
Another non-pharmacological method is the ShotBlocker® application. Based on the gate control theory, pain symptoms caused by injection are blocked temporarily. ShotBlocker® (manufactured by Bionix; Toldeo, OH) is a non-invasive, small, flat, horseshoe-shaped, yellow-colored plastic device that does not cause any adverse effects, is suitable for all age groups, and does not carry drug properties. ShotBlocker® ready to use immediately, and there is no need to wait for to the take effect (http://www. bionixmed.com/) (Figure 1).
Figure 1: Determine the effects of ShotBlocker®.
There are many studies examining the effects of the ShotBlocker® on pain related to injection in adults and children [5,22,26,27]. However, in the last 5 years, only 4 studies in children (vaccination=1, Intramuscular (IM) injections=2, insulin injection=1) have been limited to the effectiveness of the ShotBlocker® on pain [5,21,22,28]. Recently, Sahan and Yildiz published a meta-analysis of the effects of the ShotBlocker® application on intramuscular injection pain in adults, and it has been found to be effective in reducing pain [27].
The questions of this meta-analysis and systematic review:
1. What is the effect of ShotBlocker® in reducing pain of injections in children?
2. What is the effect of ShotBlocker® in reducing fear/anxiety/, distress and/or stress of injections in children?
This study was conducted to determine the effects of ShotBlocker®, application during administration of injections to children for providing an evidence-based practice.
Material and Methods
This study is a systematic review and meta-analysis. The cochrane handbook for systematic reviews of interventions’ guidelines [29].
Search strategy
No restriction on publication date was imposed but the publication languages were restricted to English, Turkish and German. The literature search was conducted from April 1, 2021 to June 31, 2021 in PubMed, EBSCO via Medline, EBSCO via Cinahl, Cochrane Trials, Web of Science, and Care Lit. Gray literature was searched via Google Scholar and BIONIX (manufacturer ShotBlocker®). Study protocols were searched via Cochrane Protocols, clinicaltrailregister. eu and clinicaltrails.gov.
The search terms “ShotBlocker®”, “Gate control theory”, “pain”, “child” as well as known synonyms and database-specific search terms (including Mesh) were used. A draft of the search terms and strategy used for one of the databases is presented in the Appendix.
A total of 339 publications were identified of these, 183 were duplicates. After title and abstract screening, using the inclusion and exclusion criteria, 10 publications were included in the full-text screening (Table 1). The title and abstract screening was carried out independently by the authors BBS and MB and a consensus was reached between the authors in the event of discrepancies. We scanned the entire literature until the time we wrote the article, without time limits.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Children (0-18 years) | Adults |
| Procedural Pain Procedural Fear/Anxiety | Other types of pain (migraine, tumor pain, postoperative pain) |
| Randomized controlled trials | Biochemical studies of basic research |
| Animal studies |
Table 1: Inclusion and exclusion criteria
The Risk of Bias (RoB-2) instrument was then used to check for biases of the ten identified studies (Figure 1). RoB2 is a wildly used tool for the assessment of bias in Randomized Controlled Trial (RCTs). It is easy to use and represents large reliability and validity. For visualization of the bias risk‐of‐bias visualization (robvis) was used [30]. Authors BBS and MB conducted the reviews independently. In case of disagreement, a consensus was tried to be established with the help of the guide, and if this was not successful, the third author Cornelia Mahler was consulted. The results are summarized in Table 2.
| Study | Faces Pain Scale – Revised (FPS-R) | Visual Analog Scale (VAS) | Wong- Baker FACES-Pain Scale | Neonatal Infant Pain Scale (NIPS) | 6 point Likert Scale | Oucher Scale | Numerische Rating-Skala (NRS) | Children’s Fear Scale (CFS) | State-Trait Anxiety Inventory for Children (STAIC) | Children`s Anxiety and Pain (CAPS) | Children's heart/puls Rate | Children's Respiratory Rate | Behavioral Scale |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bilgen, 2019 | X | X | - | - | - | - | - | - | X | - | - | - | - |
| Caglar, 2017 | - | - | - | X | - | - | - | - | - | - | X | X | - |
| Cobb, 2009 | X | X | - | - | - | - | - | - | - | - | X | - | X |
| Drago, 2009 | - | - | X | - | X | - | - | - | - | - | - | - | |
| Girgin, 2020 | - | - | X | - | - | - | - | X | - | - | - | - | - |
| Guevarra, 2003 | - | - | X | - | - | - | - | - | - | - | - | - | - |
| Gundrum, 2001 | - | - | - | - | - | - | X | - | - | - | - | - | - |
| Mennuti-Washburn, 2007 | X | X | - | - | - | - | - | - | -- | - | X | - | - |
| Sahiner, 2018 | X | - | - | - | - | - | - | - | - | X | - | - | - |
| Yilmaz, 2019 | - | - | - | - | - | X | - | X | - | - | - | - | - |
Table 2: Outcomes of studies
Data extraction and data analysis
In a first step, the data from the identified full texts were first extracted narratively (Author, intervention, year, number of participants). An attempt was made to pool the results and summarize them in a meta-analysis. Data analyses were conducted using the Review Manager for all outcomes (Rev Man, version 5.4, Cochrane Collaboration, and London). Standardized Mean Differences (MD) with 95% Confidence Intervals (CI) was calculated. The significance level of the statistical analysis was determined as 0.05 in this meta-analysis. The I2 value was used to determine the degree of heterogeneity between the studies. Finally, the results were summarized in a short practical recommendation.
Outcomes
The primary outcomes of our study are pain and fear/anxiety evaluated either before, during or after the pain procedure. The following comparisons were done:
•ShotBlocker® vs. cold and vibration device
• Children self-reported pain
• Observer-reported pain
• Children self-reported fear/anxiety
• Observer-reported fear/anxiety
•ShotBlocker®vs. usual care
• Children self-reported pain
• Observer-reported pain
• Children self-reported fear/anxiety
• Observer-reported fear/anxiety
•ShotBlocker® vs. Placebo
• Children self-reported pain
• Observer-reported pain
• Children self-reported fear/anxiety
• Observer-reported fear/anxiety
Results
Sampling
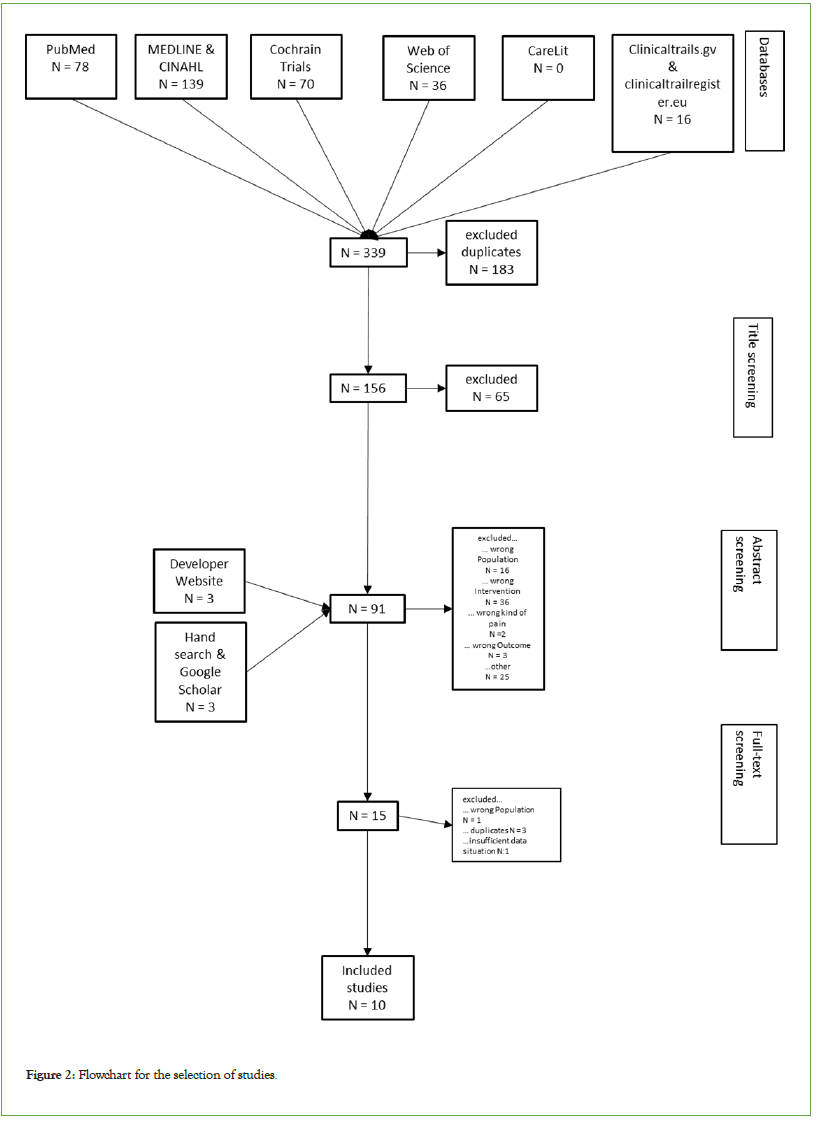
The initial search identified eleven studies, but not all studies were feasible for meta-analysis. The sampling and screening procedures are summarized in the flow diagram (Figure 2). A total of eleven trials encompassing 1121 participants were included in the analysis [5,21,22,28,31-36].
Figure 2: Flowchart for the selection of studies.
General characteristics
In total, eleven studies involving a total of 1121 participants. The baseline characteristics of the eleven publications are summarized in Table 3. Five of the included trials were from Turkey, four from USA, and one from the Philippines [5,21,22,28,31-36]. Eight of the included trials recruited children aged from two month to seventeen years, while one trial recruited full-term neonates [5,21,22,28,31-33,35,36].
| Autor | Country | Sample | Age | Intervention | Control | Outcomes | Assesment instrument | Results (Numbers) | Results (Verbal) | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| Bilgen Sivri & Balci (2019) | Turkey | 150 | 7-12 a | ShotBlocker® | Buzzy® | Anxiety | STAIC | STAIC: Mean +/- Standard Diviation (before the procedure) SB=38,50 +/- 5.47; B=37,74 +/- 6,07; C=40,16 +/- 6,24 (p>0,5). VAS 1 min. Mean +/- Standard Diviation SB=6,36 +/- 3,24; B=3,68 +/- 3,05; C=7,34 +/- 3,11 (p<0,001) VAS 5 min Mean +/- Standard Diviation SB=3,38 +/- 2,94; B=1,68 +/- 2,28; C=4,88 +/-3,24 (p<0,001) Mean Score Difference Mean +/- Standard Diviation SB=-2,90+/- 1,59; B=-2,00 +/- 1,67; C=-2,46 +/- 1,69 (p=0,009) FPS-R 1 min Mean +/- Standard Diviation SB=6,24 +/- 3,20; B=3,64 +/- 3,10; C=7,36 +/- 3,09 (p<0,001) FPS-R 5 Min Mean +/- Standard Diviation SB=3,24 +/- 2,96; B=1,52 +/- 2,23; C=4,84 +/- 3,29 (p<0,001) Mean Score Difference Mean +/- Standard Diviation SB= -2,92 +/-1,48; B= -2,12 +/- 1,73; C= -2,52 +/- 1,71 (p=0,012) | SB significantly better than C; B significantly better than SB and C | |
| Usual care | Pain | VAS | ||||||||
| FPS-R | ||||||||||
| Caglar et al., (2017) | Turkey | 100 | Full-term neonates | ShotBlocker® + swaddling | Usual Care (Swaddling) | Pain | NIPS (0-7) | NIPS preinjection Mean +/- Standard Diviation SB=0,62 +/- 0,83; C=0,70 +/- 0,81; (p=0,631) NIPS injection Mean +/- Standard Diviation SB=1,64 +/- 0,80; C=2,96 +/- 0,73; (p= ≤ .01) NIPS postinjection Mean +/- Standard Diviation SB=0,74 +/- 0,66; C=1,42 +/- 0,76; (p= ≤ .01) Heart rate preinjection Mean +/- Standard Diviation SB=137,26 +/- 12,64; C=140,68+/- 11,82; (p=0,165) Puls rate postinjection Mean +/- Standard Diviation SB=145,02 +/- 13,50; C=150,24 +/- 13,36; (p= ≤.05) Respiratory rate preinjection Mean +/- Standard Diviation SB=57,28 +/- 7,52; C=55,68 +/- 7,79; (p=0,299) Respiratory rate postinjection Mean +/- Standard Diviation SB=59,96 +/- 7,20; C=59,04 +/- 8,15; (p=0,552) | SB significantly better on NIPS and puls rate | ShotBlocker® takes significantly longer (p=0,000) |
| Puls rate | ||||||||||
| Respiratory rate | ||||||||||
| Cobb & Cohen (2009) | England | 89 | 4-12 a | ShotBlocker® | Placebo | Pain | FPS-R (0-10 points) | FPS-R Mean +/- Standard Diviation Self SB=4,25 +/- 3,55; P=4,00 +/- 4,20; C=4,67 +/- 3,68 VAS Parents (Pain)Mean +/- Standard Diviation SB=36,83 +/- 26,73; P=37,44 +/- 34,53; C=37,81 +/- 33,21 VAS Nurse (Pain) Mean +/- Standard Diviation SB=38,28 +/- 28,89; P=34,39 +/- 26,94; C=36,03 +/- 26,34 Heart-rate change Mean +/- Standard Diviation SB=-4,78 +/- 32,63; P=6,33 +/- 17,92; C=3,14 +/- 17,50. Behavior preinjection Mean +/- Standard Diviation SB=0,14 +/- 0,26; P=0,05 +/- 0,20; C=0,08 +/- 0,18 Behavior injection Mean +/- Standard Diviation SB=0,41 +/- 0,46; P=0,26 +/- 0,38; C=0,22 +/- 0,39; Behavior postinjection Mean +/- Standard Diviation SB=0,27 +/- 0,37; P=0,20 +/- 0,33; C=0,20 +/- 0,38 VAS Parents (Anxiety) Mean +/- Standard Diviation SB=59,62 +/- 36,16; P=57,75 +/- 37,40; C=54,29 +/- 37,90 VAS Nurse (Anxiety) Mean +/- Standard Diviation SB=48,10 +/- 39,31; P=54,93 +/- 29,24; C=50,84 +/- 33,63 | No significant difference between groups however no p values given | |
| Usual care | Anxiety | Heart-rate | ||||||||
| Parents VAS | ||||||||||
| Behavior scale Shouting, crying or restraint from adult | ||||||||||
| VAS for Anxiety in Parents and Observers | ||||||||||
| Drago et al., (2009) | USA | 165 | 2 m -17 a | ShotBlocker® | Usual care | Pain | 6 point Likert scale | Perceived pain score: nurses Mean +/- Standard Diviation C: 2.6 +/- 1.5; SB=1.8 +/-1.3 (p=0.001) Perceived pain score: parents Mean +/- Standard Diviation C: 2.6 +/- 1.7; SB: 2.1 +/- 1.6; (p=0.045) Pain score from all children able to complete a Baker Wong pain assessment Children ≥36 mo Mean +/- Standard Diviation C (n=28):1.5 +/- 1.6; SB (n=36) : 1.3 +/- 1.5; (p=0 .7) Pain score from older children Children ≥72 mo Mean +/- Standard Diviation C (n=14): 1.3 +/- 1.4; SB (n=18): 0.5 +/- 0.8; (p=0.04) | Observer assessment significantly better for SB than for C In children over 6 years SB significantly better than C | Difficulties of nursing in the application with 6 point Likert scale mean = 1.7 no SD and p level given |
| Wong- Baker FACES-Pain Scale (0-5 points) | ||||||||||
| Girgin Aykanat et al., (2020) | Turkey | 90 | 6-12 a | ShotBlocker® | Buzzy® | Pain | Wong- Baker FACES-Pain Scale (0-10 points) | Wong- Baker FACES-Pain Scale (Children) Mean +/- Standard Diviation SB=1,23 +/- 0,68; B=0,23 +/- 0,50; C=3,00 +/- 0,91 (p=0,0001) Wong- Baker FACES-Pain Scale (Parent) Mean +/- Standard Diviation SB=1,20+/- 0,66; B=0,20+/- 0,48; C=3,00 +/- 0,87 (p=0,0001) Wong- Baker FACES-Pain Scale (Researcher) Mean +/- Standard Diviation SB=1,27 +/- 0,64; B=0,20 +/- 0,48; C=2,97 +/- 0,81 (p=0,0001) CFS (Children) Mean +/- Standard Diviation SB=1,17 +/- 0,75; B=0,20 +/- 0,41; C=2,93 +/- 0,78 (p=0,0001) CFS (Parent) Mean +/- Standard Diviation SB=1,23 +/- 0,73; B=0,27 +/- 0,45; C=3,03 +/- 0,61 (p=0,0001) CFS (Researcher) Mean +/- Standard Diviation SB=1,20 +/- 0,76; B=0,23 +/- 0,43; C=3,03 +/- 0,81 (p=0,0001) | Significant only post injection Significant reduction in all actors B >SB>C in fear and pain | Parent satisfaction highest at B lowest at C (p=0.0001) |
| Usual care | Fear | CFS (0-4 points) | ||||||||
| Gundrum (2001) | USA | 99 | 5 through adult | ShotBlocker® | Usual Care | Pain | NRS | - | Significant lower pain in the ShotBlocker® group | |
| Guevarra (2003) | Philippinen | 119 | 5 -6 a | ShotBlocker® | Usual Care | Pain | Wong- Baker FACES-Pain Scale (0-10 points) | Wong- Baker FACES-Pain Scale Mean +/- Standard Diviation SB=0,3 +/-0,6; C=1,3+/- 1,1 (p<0,001) | ||
| Mennuti-Washburn(2007) | USA | 89 | 4 -12 a | ShotBlocker® | ShotBlocker®-placebo | Pain | Children: FPS-R | - | No significant difference in child's pain perception; PP and parents | |
| Usual-care | Anxiety | Heart-rate | ||||||||
| Parents: VAS Nurse: VAS | ||||||||||
| Sahiner et al., (2018) | Turkey | 60 | 6 -12 a | ShotBlocker® | Buzzy® | Pain | CAPS (1-5 pionts) | FPS-R Children Mean +/- Standard Diviation SB=0,90 +/- 1,20; B=1,26 +/- 1,36; C=3,2 +/- 2,78; (p=0,008) FPS-R Parents Mean +/- Standard Diviation SB=1,10 +/- 1,51; B=2,20 +/- 1,82; C=3,50 +/- 2,74; (p=0,007) FPS-R Observer Mean +/- Standard Diviation SB=0,90 +/- 1,20; B=1,20 +/- 1,19; C=3,30 +/- 2,27; (p=0,000) CAPS Parents Mean +/- Standard Diviation SB=0,25 +/- 0,55; B=0,90 +/- 1,07; C=1,35 +/- 1,38; (p=0,006) CAPS Observer Mean +/- Standard Diviation SB=0,15 +/- 0,48; B=0,60 +/- 1,14; C=1,30 +/- 1,41; (p=0,002) | SB significantly better than C and B for anxiety and pain | SB 0,005$ per use B 0,09$ per use |
| Usual Care | Anxiety | FPS-R (0-10) | ||||||||
| Yilmaz et al., (2019) | Turkey | 160 | 5-10 a | ShotBlocker® | Buzzy® | Pain | CFS (0-4 points) | Oucher Children Mean +/- Standard Diviation SB=4,14 +/- 2,12; B=3,87 +/- 1,79; BB=4,75 +/- 1,74; C=6,72 +/-2,16; (p=0,02) Oucher Parents Mean +/- Standard Diviation SB=4,51 +/- 3,49; B=3,18 +/- 2,85; BB=5,65 +/- 3,26; C=6,85 +/- 2,64 (p=0,03) Oucher Observer Mean +/- Standard Diviation SB=4,23 +/- 3,56; B=3,09 +/- 3,08; BB=5,13 +/-3,15; C=6,30 +/- 4,09 (p=0,01) CFS (Children) Mean +/- Standard Diviation SB=1,66 +/- 0,53; B=1,35 +/- 0,60; BB=1,88 +/- 0,61; C=2,82 +/- 0,66; (p=0,02) CFS (Parents) Mean +/- Standard Diviation SB=1,65 +/- 0,44; B=1,38 +/- 0,56; BB=1,93 +/- 0,53; C=2,85 +/- 0,74 (p=0,01) CFS (Observer) Mean +/- Standard Diviation SB=1,57 +/- 0,53; B=1,39 +/- 0,49; BB=1,74 +/- 0,57; C=2,60 +/- 0,70 (p=0,03) | Significant difference between pain perception ShotBlocker®/ Control by all observers; Significant difference between Buzzy® and ShotBlocker® (Buzzy® better) observed by parent and observer and ShotBlocker® control related to anxiety observed by child, parent and observer B >SB >BB >C | |
| Bubble-blowing | Oucher-Scala (0-10 points) | |||||||||
| Usual-care | Fear |
Table 3: Description of included studies.
The needle-related procedures only include two procedures IM injection/vaccination (n=9) and subcutaneous (sc) insulin injection (n=1) [5,21,22,28,31-36].
To assess pain, the following scales were applied: The Faces Pain Scale-Revised (FPS-R), Visual Analog Scale (VAS), Wong-Baker FACES-Pain Scale, Neonatal Infant Pain Scale (NIPS), 6 point Likert scale, Oucher Scale, and the Numerische Rating-Skala (NRS) [5,21,22,28,31-36].
The following instruments were used to assess fear/anxiety: Children’s Fear Scale (CFS), State-Trait Anxiety Inventory for Children (STAIC), and the Children’s Anxiety and Pain Scale (CAPS) [5,22,28,36].
In addition, the following parameter were collected; Children's heart/pulse rate, and the Behavioral scale, respiratory rate [21,31,35].
In the included studies, the following non-pharmacological methods (Buzzy®, swaddling, bubble-blowing) were used to compare with ShotBlocker® [5,21,22,28,31-33,35,36].
The meta-analysis was carried out with eight out of ten studies and is also shown in the results [5,21,22,28,31-33,36]. For some subcategories, a meta-analysis is not helpful because only one original study examines this perspective [31]. Therefore, forest lands are not included for all subcategories.
Risk of bias
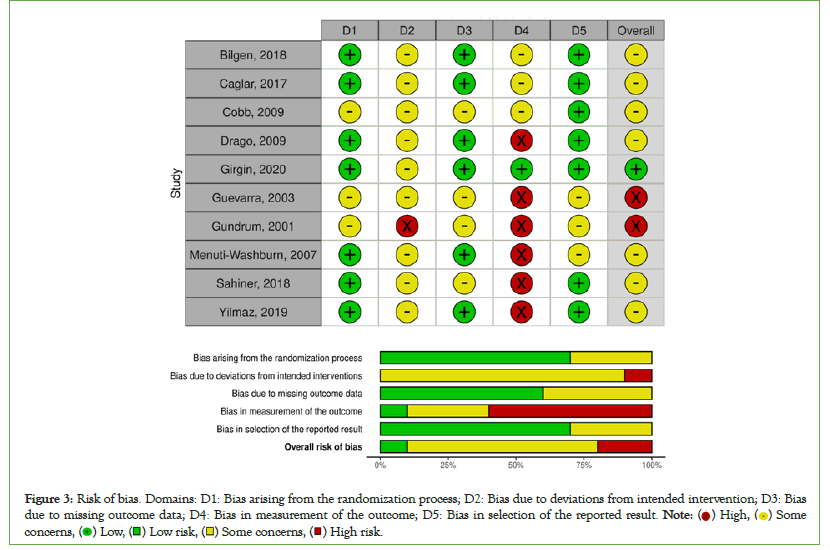
The results of assessment of risk of bias of the included trials are delineated in Figure 3. Overall, the included studies have a moderate to high risk of bias. The strongest biases in the included studies are reporting and detection bias. It should be noted that blinding of the participants and parents was not possible and in most cases the nurses performing the tests were not blinded either.
Figure 3: Risk of bias. Domains: D1: Bias arising from the randomization process; D2: Bias due to deviations from intended intervention; D3: Bias due to missing outcome data; D4: Bias in measurement of the outcome; D5: Bias in selection of the reported result. Note: ( ) High, (
) High, ( ) Some concerns, (
) Some concerns, ( ) Low, (
) Low, ( ) Low risk, (
) Low risk, ( ) Some concerns, (
) Some concerns, ( ) High risk.
) High risk.
ShotBlocker® vs. cold and vibration device: These are the following
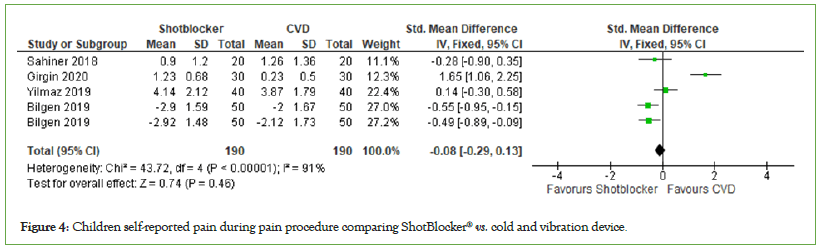
Children self-reported pain:Four trials reported pain observed by children, with a total number of participants of 380, and these were used in the meta-analysis. Meta-analysis of these trials showed that children treated with cold and vibration devices had slight reduction in pain compared with the ShotBlocker® devices, but it was not significant. (MD=−0.08, 95% CI: −0.29, 0.13, I2=91%, P=0.46) (Figure 4).
Figure 4: Children self-reported pain during pain procedure comparing ShotBlocker® vs. cold and vibration device.
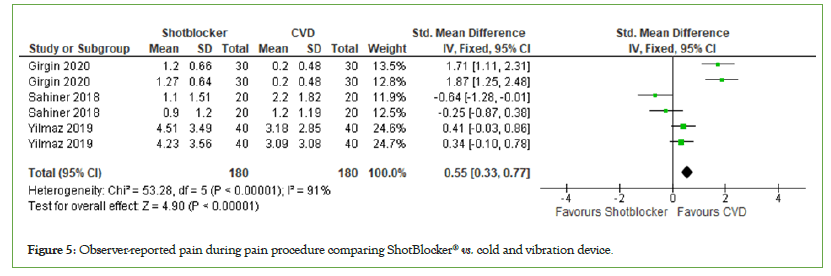
Observer-reported pain: Three trials reported pain observed by observer, with a total number of participants of 360. Meta-analysis shows that the observer of the intervention rated the pain lower in the children obtaining cold and vibration device as a intervention (MD=0.55, 95% CI: 0.33, 0.77, I2=91%, P<0.00001) (Figure 5).
Figure 5: Observer-reported pain during pain procedure comparing ShotBlocker® vs. cold and vibration device.
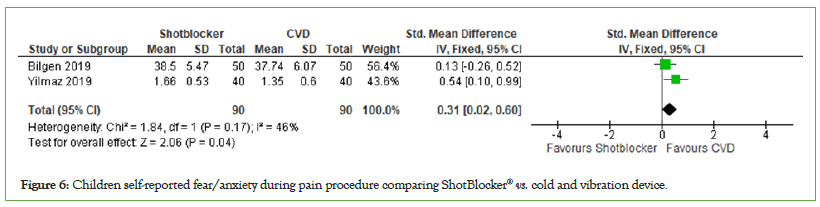
Children self-reported fear/anxiety: Two trials reported fear self- reported by the children, with a total number of participants of 180. (MD=0.31, 95% CI: 0.02, 0.60, I2=46%, P=0.17) (Figure 6). The findings in this category indicate that there is no significant difference in the performance of cold-vibration and ShotBlocker® on the self-reported fear/anxiety of the involved children.
Figure 6: Children self-reported fear/anxiety during pain procedure comparing ShotBlocker® vs. cold and vibration device.
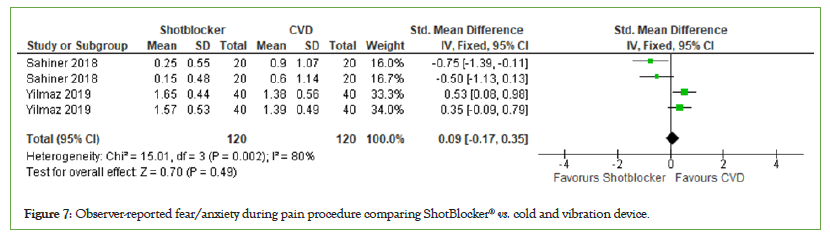
Observer-reported fear/anxiety: A total of two studies with 240 participants were included in the analysis of the effect of the ShotBlocker® device on observer-reported fear/anxiety. The data shows a slight favor for cold and vibration device but this is not significant (MD=0.09, 95% CI: −0.17, 0.35, I2=80%, P=0.49) (Figure 7).
Figure 7: Observer-reported fear/anxiety during pain procedure comparing ShotBlocker® vs. cold and vibration device.
ShotBlocker® vs. usual care: These are the following
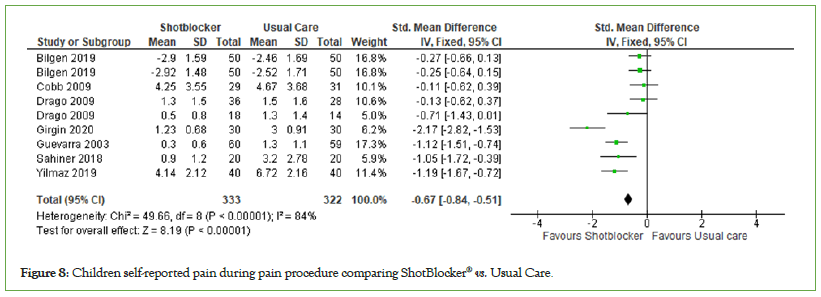
Children self-reported pain: A total of seven studies with 655 participants (ShotBlocker®=333, Usual Care=322) were included in the analysis of the effect of the ShotBlocker® device on children self-reported pain. The overall effect was found to be significant (MD=−0.67, 95% CI: −0.84, −0.51, I2=84%, P<0.00001) (Figure 8). The finding indicate that ShotBlocker® is significant better in reducing self-reported pain in children then the usual care approach.
Figure 8: Children self-reported pain during pain procedure comparing ShotBlocker® vs. Usual Care.
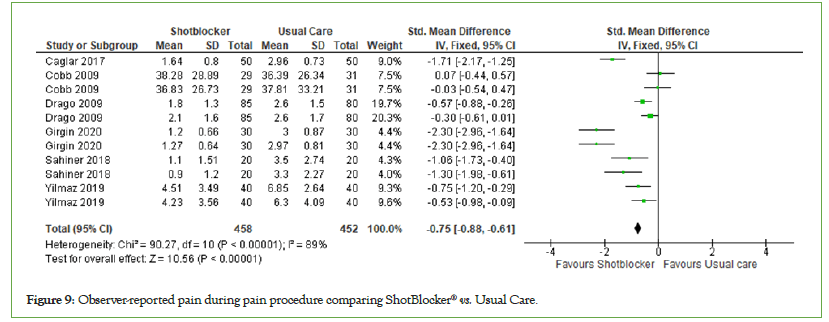
Observer-reported pain: Six studies examined the effect of the ShotBlocker® device on observer-reported pain. The total number of participants was 458 in the ShotBlocker® group and 452 in the usual care group (n=910). The observer-reported pain was significantly lower for the ShotBlocker® device than for the usual care with (MD=−0.75, 95% CI: −0.88, −0.61, I2=89%, P<0.00001) considerable heterogeneity (Figure 9).
Figure 9: Observer-reported pain during pain procedure comparing ShotBlocker® vs. Usual Care.
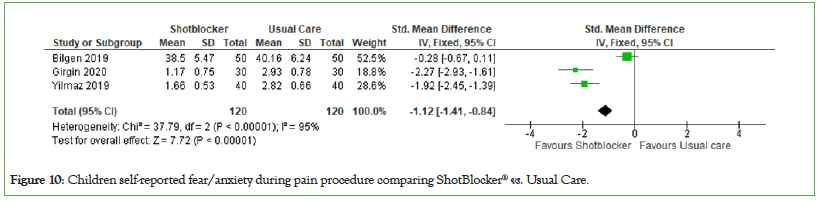
Children self-reported fear/anxiety: The effects of the ShotBlocker® device on children-reported fear were investigated in 3 studies with a total of 240 participants (ShotBlocker®=120, Usual care=120). Analyses suggest that the effect of this device is significant for reducing children fear during needle-related procedures compared to usual care (MD=−1.12, 95% CI: −1.41, −0.84, I2=95%, P<0.00001) (Figure 10).
Figure 10: Children self-reported fear/anxiety during pain procedure comparing ShotBlocker® vs. Usual Care
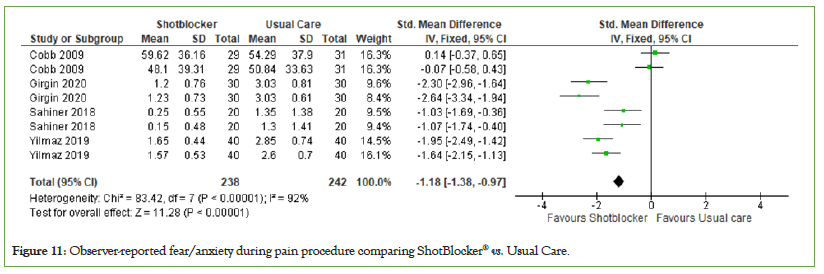
Observer-reported fear/anxiety: Four trials reported children's fear observed by observer during the needle procedures. The total number of participants was 480. Meta-analysis shows that the observer reported pain in children receiving ShotBlocker® as an intervention is significant lower than the group receiving usual care (MD=−1.18, 95% CI: −1.38, −0.97); significant heterogeneity was observed across trials (P<0.00001, I2=92%) (Figure 11).
Figure 11: Observer-reported fear/anxiety during pain procedure comparing ShotBlocker® vs. Usual Care.
ShotBlocker® vs. placebo: Because only one study took a closer look at the comparison with a placebo, no meta-analysis was possible [31]. But the study suggested that there was no difference between the two groups in the outcome of self-reported pain, observer reported pain and observer reported fear/anxiety. At the same time, although it was stated in Mennuti-Washburn study that pain and fear/anxiety were evaluated according to the statements of children, caregivers and health personnel between ShotBlocker® and placebo, meta-analysis could not be performed because no values (Mean/Standard Deviation) were obtained [35].
Discussion
To our knowledge, this is the first systematic review and metaanalysis assessing the combining effects of the ShotBlockerShotBlocker® device for needle related pain and fear/anxiety in children. This review synthesizes the evidence of 10 RCTs, of which 8 were suitable for meta-analysis. The meta-analysis compared the ShotBlocker® to cold and vibration device, Usual Care, and Placebo for reducing pain (self/observer-reported) and fear/anxiety (self/observer-reported). The results provide a detailed summary of the evidence for its use in children of needle-related procedures. The strengths and limitations of the included studies and this review will be discussed, and recommendations for further clinical practice and RCTs will be presented.
Painful procedures are one of the important topics studied in children and there is constant research on the subject. Two nonpharmacologic methods used to reduce pain during procedures are ShotBlocker® and Buzzy® (cold-vibration) devices. In these methods, according to the door control theory, the pain signals of the injection are blocked temporarily, and the “doors” leading to the central nervous system are closed [18]. In this study, investigated the effect of ShotBlocker® VS. Cold and vibration device in reducing pain and fear/anxiety in painful procedures [5,22,28,36]. According to the researchers, when examining the mean fear/ anxiety scores of children in the ShotBlocker®, and cold-vibration device groups before the procedure in studies, the children had similar mean fear/anxiety scores and the difference between the groups was not statistically significant. In three studies, children were given intramuscular injection and it was found that pain and fear/anxiety were lower in the cold and vibration group after the procedure [5,36]. However, Sahiner et al., used the cold-vibration device and ShotBlocker® receiving insulin injections in children and had lower fear/anxiety (parent, child, and observer) and pain (parent, observer) scores than cold-vibration device group [22]. Considering the number of studies, we may recommend more studies to be carried out on the effect of ShotBlocker® and coldvibration device application at different sites.
At the same time, in this review compared the ShotBlocker® device with a Usual Care and the effect of the device was significant in reducing self-reported procedural pain, observer-reported pain, self-reported procedural fear/anxiety, observer-reported fear/ anxiety. In a study conducted by Drago et al., on 165 children in the USA, ShotBlocker® was used while injecting children with IM and pain scores decreased according to the evaluations of nurses and caregivers, but no difference was found according to the evaluations of the children [32]. However, when a subgroup of slightly older children was examined (children ≥ 6 years of age), the pain scores in the ShotBlocker® group were lower than those in the standard of care group. In a study of 119 preschool children, those children who were given ShotBlocker® during IM injections had significantly less pain compared with the control group [33]. In their study, Gundrum et al., found that ShotBlocker® was effective in alleviating acute pain in children (over 5 years old) who received IM vaccination [34]. However, Mennuti-Wasburn and Cobb et al., used ShotBlocker® while IM vaccinating 4 to 12 years children and reported that it was not effective in reducing pain [31,35]. It suggests that the differences between the results of the studies may be due to the fact that the studies were conducted in different age groups and different muscle groups.
When the literature is reviewed, many other non-pharmacological approaches including methods such as swaddling, bubble blowing, therapeutic clowning, music, and using distraction cards are used in painful procedures, as well as ShotBlocker® [10,11,14,21]. In fact, it has been observed that these methods are used together in some studies. In our study, Caglar et al., administered IM hepatitis B vaccine in 100 full-term neonates (ShotBlocker®+swaddling, Usual care (swaddling)) [21]. The pain scores of the neonates during and after the injection procedure were lower in the ShotBlocker® group than in the control group. The post injection heart rate in the infants in the ShotBlocker® group was found to be lower than in those for whom ShotBlocker® was not used. Although various methods have been used in studies to reduce pain and fear/ anxiety in needle procedures, the effectiveness of a single method cannot be highly recommended. Whether a distraction method such as swaddling should be offered as an option in addition to the ShotBlocker® method with needle procedure is an interesting question in terms of practical impact, time, cost, and staff training [37,38]. More trials comparing the two methods are needed.
According to the systematic review and meta-analysis, we recommend that further randomized controlled trials are conducted concerning the efficiency of ShotBlockerShotBlocker® in reducing pain induced by needlerelated procedures in children.
Conclusion
According to result of the systematic review and meta-analysis, ShotBlocker® treatment was found to reduce pain and fear/anxiety levels in children who received injections in some studies, while Buzzy® was found to be more effective in some studies. As seen in the articles discussed, it can be said that non-pharmacological methods such as ShotBlocker® and Buzzy® are used in reducing pain and fear/ anxiety, it is difficult to say which is more effective, and therefore further randomized controlled studies are needed. Although ShotBlockerShotBlocker® is effective in reducing pain and fear/anxiety in some studies and not effective in some others, it is non-invasive; there is no need to wait for to the take effect, and harmless device.
It can also help parents and other observers to cope with the inflicted pain. Therefore we can promote the use of ShotBlocker® as a pain management utensil in procedural related pain in children, over usual care.
Adverse events
Descriptions regarding adverse events were not reported in included studies. ShotBlocker® device was well tolerated.
Limitations
This systematic review presents four potential limitations. In this study, only randomized controlled studies in English, Turkish and German were searched. If there is a study in another language, it could not be reached. Additionally, we did not contact experts in the field to investigate whether they had unpublished studies and there was a lack of protocol registration (clinical trial number). The meta-analysis was fully performed, but when the subgroups were examined, meta-analysis could not be done because there were only two studies comparing ShotBlocker® and placebo, and values were not given in one study. We did not consider the contextual issues and culture that might have influenced the perception of children’s pain and fear/anxiety and the parents’/observers’ assessment of children’s pain, as we did not have enough data to address this issue. Many different assessments were used to determine pain or fear/anxiety therefore it could be possible, do to cutoff point, sensitivity and validity, that the performed meta-analysis is not as precise as it seems.
Implications for practice
• Health professionals should be aware of pain during needlerelated procedure and use methods for pain and fear/anxiety relief accordingly.
• ShotBlocker® is recommended as a helpful option in cases where a pain and fear/anxiety relief method is required.
Contribution to authorship
CM, BBS, and MB designed the research. The literature review was carried out independently by BBS and MB, risk of bias was assessed by BBS and MB and data analysis was carried out by MB. CM, BBS and MB contributed to subsequent titles and approved the final article.
Declaration of competing interest
The authors declare no conflicts of interests relevant to this article.
References
- IASP-International Association for the Study of Pain. IASP Announces revised definition of pain. 2020.
- Cirik VA, Çiftçioğlu Ş, Efe E. Knowledge, practice and beliefs of pediatric nurses about pain. J Pediatr Res. 2019;6(3): 220-227.
- Krauss BS, Calligaris L, Green SM, Barbi E. Current concepts in management of pain in children in the emergency department. The Lancet. 2016;387(10013):83-92.
[Crossref] [Google Scholar] [PubMed]
- McMurtry CM, Riddell RP, Taddio A, Racine N, Asmundson GJ, Noel M, et al. Far from “just a poke”: Common painful needle procedures and the development of needle fear. Clin J Pain. 2015;31:S3-S11.
[Crossref] [Google Scholar] [PubMed]
- Sivri Bilgen B, Balci S. The effect on pain of BuzzyShotBlocker® and ShotBlockerShotBlocker® during the administration of intramuscular injections to children: A randomized controlled trial. J Korean Acad Nurs. 2019;49(4):486-494.
[Crossref] [Google Scholar] [PubMed]
- Ortiz MI, López‐Zarco M, Arreola‐Bautista EJ. Procedural pain and anxiety in paediatric patients in a Mexican emergency department. J Adv Nurs. 2012;68(12):2700-2709.
[Crossref] [Google Scholar] [PubMed]
- Schechter NL, Zempsky WT, Cohen LL, McGrath PJ, McMurtry CM, Bright NS. Pain reduction during pediatric immunizations: Evidence-based review and recommendations. Pediatrics. 2007;119(5):e1184-e1198.
[Crossref] [Google Scholar] [PubMed
- Törüner EK, Büyükgönenç L. Child Health Basic Nursing Approaches. Ankara: Göktuğ Publishing. 2017;146-171.
- Basiri-Moghadam M, Kianmehr M, Pasban-Noghabi S, Basiri-Moghadam K. Comparison of EMLA cream with rattles on reducing immunization pain in four months infants. J Pak Med Assoc. 2014;64(8):874-878.
[Google Scholar] [PubMed]
- Tork HM. Comparison of the effectiveness of Buzzy®, distracting cards and balloon inflating on mitigating pain and anxiety during venipuncture in a pediatric emergency department. Am J Nurs Sci. 2017;6(1):26-32.
- Kurudirek F, Arıkan D. Effects of therapeutic clowning on pain and anxiety during intrathecal chemotherapy in Turkey. J Pediatr Nurs. 2020;53:e6-e13.
[Crossref] [Google Scholar] [PubMed]
- Canbulat N, Inal S, Sönmezer H. Efficacy of distraction methods on procedural pain and anxiety by applying distraction cards and kaleidoscope in children. Asian Nurs Res Sci. 2014;8(1):23-28.
[Crossref] [Google Scholar] [PubMed]
- Akgül EA, Karahan Y, Başoğlu F, Oğul A, Öztornaci BÖ, Yetim P, et al . Effects of watching cartoons on pain scores in children undergoing venipuncture. Nurs Child Young People. 2018;pp.1-6.
- Johnson AA, Berry A, Bradley M, Daniell JA, Lugo C, Schaum-Comegys K, et al. Examining the effects of music-based interventions on pain and anxiety in hospitalized children: An integrative review. J Pediatr Nurs. 2021;60:71-76.
[Crossref] [Google Scholar] [PubMed]
- Koç T, Gözen D. The effect of foot reflexology on acute pain in infants: A randomized controlled trial. Worldviews Evid Based Nurs. 2015;12(5):289-296.
[Crossref] [Google Scholar] [PubMed]
- Lander J, Fowler-Kerry S. TENS for children's procedural pain. Pain. 1993;52(2):209-216.
[Crossref] [Google Scholar] [PubMed]
- Koç Özkan T, Balcı S. The effect of acupressure on acute pain during venipuncture in children: Implications for evidence‐based practice. Worldviews Evid Based Nurs. 2020;17(3):221-228.
[Crossref] [Google Scholar] [PubMed]
- Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965;150(699):971-979.
- Inal S, Kelleci M. The effect of external thermo-mechanical stimulation and distraction on reducing pain experienced by children during blood drawing. Pediatr Emerg Care. 2020;36(2):66-69.
[Crossref] [Google Scholar] [PubMed]
- Ballard A, Khadra C, Adler S, Trottier ED, Le May S. Efficacy of the Buzzy® device for pain management during needle-related procedures. Clin J Pain. 2019;35(6):532-543.
[Crossref] [Google Scholar] [PubMed]
- Caglar S, Büyükyilmaz F, Cosansu G, Çaglayan S. Effectiveness of ShotBlocker® for immunization pain in full-term neonates: A randomized controlled trial. J Perinat Neonatal Nurs. 2017;31(2):166-171.
[Crossref] [Google Scholar] [PubMed]
- Canbulat Sahiner N, Turkmen AS, Acikgoz A, Simsek E, Kirel B. Effectiveness of two different methods for pain reduction during insulin injection in children with Type 1 diabetes: Buzzy® and ShotBlocker®. Worldviews Evid Based Nurs. 2018;15(6):464-470.
[Crossref] [Google Scholar] [PubMed]
- Ballard A, Khadra C, Adler S, Doyon-Trottier E, Le May S. Efficacy of the BuzzyShotBlocker® device for pain management of children during needle-related procedures: A systematic review protocol. Syst Rev. 2018;7(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Su HC, Hsieh CW, Lai NM, Chou PY, Lin PH, Chen KH. Using vibrating and cold device for pain relievers in children: A systematic review and meta-analysis of randomized controlled trials. J Pediatr Nurs. 2021;61:23-33.
[Crossref] [Google Scholar] [PubMed]
- Ueki S, Yamagami Y, Makimoto K. Effectiveness of vibratory stimulation on needle-related procedural pain in children: A systematic review. JBI Evid Synth. 2019;17(7):1428-1463.
[Crossref] [Google Scholar] [PubMed]
- Bilge S, Aydin A, Gun C, Aldinc H, Acar YA, Yaylaci S, et al. Comparison of the efficacy of Shot Blocker and cold spray in reducing intramuscular injection-related pain in adults: A prospective, randomized, controlled trial. Saudi Med J. 2019;40(10):996-1002.
[Crossref] [Google Scholar] [PubMed]
- Şahan S, Yildiz A. The effect of ShotBlocker® application on intramuscular injection pain in adults: A meta-analysis. Clin Nurs Res. 2021;31(5):820-825.
[Crossref] [Google Scholar] [PubMed]
- Girgin BA, Aktaş E, Kılınç D, Gözen D. Let's Prefer the Pain Reducing Intervention, Buzzy® ID or ShotBlocker®: A Randomized Controlled Trial. J Behcet Uz Child Hosp. 2020;10(3):290-298.
- Higgins JP. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. 2011.
- McGuinness LA, Higgins JP. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Syn Meth. 2020:1-7.
[Crossref] [Google Scholar] [PubMed]
- Cobb JE, Cohen LL. A randomized controlled trial of the ShotBlocker® for children's immunization distress. Clin J Pain. 2009;25(9):790-796.
[Crossref] [Google Scholar] [PubMed]
- Drago LA, Singh SB, Douglass-Bright A, Yiadom MY, Baumann BM. Efficacy of ShotBlocker® in reducing pediatric pain associated with intramuscular injections. Am J Emerg Med. 2009;27(5):536-543.
[Crossref] [Google Scholar] [PubMed]
- Guevarra, MAD. Efficacy of ShotBlocker®TM in reducing pain associated with intramuscular injections in pre-school children. 2003.
- Gundrum, T. Assessment of discomfort with usual immunization practice compared to the use of usual practice and ShotBlocker®. 2001.
- Mennuti-Washburn JE. Gate control theory and its application in a physical intervention to reduce children's pain during immunization injections. 2007:pp.1-58.
- Yilmaz G, Alemdar DK. Using Buzzy®, ShotBlocker®, and bubble blowing in a pediatric emergency department to reduce the pain and fear caused by intramuscular injection: A randomized controlled trial. J Emerg Nurs. 2019;45(5):502-511.
[Crossref] [Google Scholar] [PubMed]
- Lago P, Garetti E, Bellieni CV, Merazzi D, Savant Levet P, Ancora G, et al. Systematic review of non-pharmacological analgesic interventions for common needle‐related procedure in newborn infants and development of evidence‐based clinical guidelines. Acta Paediatrica. 2017;106(6):864-870.
[Crossref] [Google Scholar] [PubMed]
- Mutlu B, Balcı S. Effects of balloon inflation and cough trick methods on easing pain in children during the drawing of venous blood samples: A randomized controlled trial. J Spec Pediatr Nurs. 2015;20(3):178-186.
[Crossref] [Google Scholar] [PubMed]
Citation: Sivri BB, Berger M, Mahler C (2023) Efficacy of the ShotBlockerShotBlocker® for Pain Management During Needle-related Procedures: A Systematic Review and Meta-Analysis. J Pain Manage Med. 9:231.
Copyright: © 2023 Sivri BB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.