Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 0, Issue 0
Effectiveness of SARSâCoVâ2 Vaccines used in Malaysia: A Systematic Review and Meta-Analysis
Anita Devi Krishnan Thantry*, Palaniappan, Edwin Cheah Shenq and DhanraajReceived: 24-Jul-2023, Manuscript No. JVV-23-22317; Editor assigned: 26-Jul-2023, Pre QC No. JVV-23-22317 (PQ); Reviewed: 11-Aug-2023, QC No. JVV-23-22317; Revised: 18-Aug-2023, Manuscript No. JVV-23-22317 (R); Published: 25-Aug-2023, DOI: 10.35248/2157-7560.14.S23.001
Abstract
Introduction: COVID-19 caused by SARS-CoV-2 was declared a pandemic by WHO in March 2020 following which many vaccines were synthesised which effectively reduced the case fatality ratio. Malaysia implemented community- wide COVID-19 vaccination using ChAdOx1 (AZD1222, Astra Zeneca), BNT162b2 mRNA (Pfizer BioNTech) and CoronaVac (Sinovac) vaccines in 2021. Efficacy was established by randomized control trials prior to its roll out, but the effectiveness of these vaccines in a real-world community setting can be assessed from hospital data and research articles published from 2021 onwards.
Aim: This study aimed to do a systematic analysis of the available articles from various countries which used the three vaccines introduced in Malaysia and compare with the outcome in Malaysia.
Methods: We performed a systematic analysis on various studies about the three vaccines from different parts of the world, based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) recommendations with well-defined inclusion and exclusion criteria. The studies for each vaccine were analysed for the Relative Risk Reduction (RRR) and vaccine effectiveness. Further, a comparison between the three vaccines was attempted and compared with the effectiveness of the three vaccines in Malaysia.
Results: BNT162b2 had an RRR ≥ 90% against severe symptomatic SARS-CoV-2 with very high effectiveness upon completion of two doses. ChAdOx1 vaccine effectiveness showed a wide range from 67.5%-95.6%. Effectiveness for CoronaVac ranged from 54%-99.9%. Meta-analysis of the data was done using Microsoft Excel. A comparison between the three vaccines showed a higher effectiveness for BNT162b2(94.9%) followed by ChAdOx1(84.7%) and SinoVac(72.4%). The effectiveness of ChAdOx1 vaccine reported from our country is the highest among the studies.
Conclusion: Real-world evidence shows that COVID-19 vaccines are highly effective against severe disease, hospitalization, and death. Our study validates the importance of assessing effectiveness of COVID vaccines in preventing severe COVID.
Keywords
SARS-CoV-2 vaccines; COVID-19; Systematic review; Meta-analysis; Effectiveness; Safety; Side effects
Introduction
COVID-19 is caused by the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was declared a global pandemic by the World Health Organization (WHO) on March 11, 2020, following the first case reported in Wuhan City, China, in December 2019 [1]. As of 20 September 2022, SARS-CoV-2 has affected more than 200 countries with 609,848,852 confirmed cases of COVID-19, including 6,507,002 deaths, reported to WHO [2].
Implementation of strict personal protective measures like wearing properly fitted masks, social distancing, staying at home, restricted travel, hand hygiene etc., played a major role in reducing transmission and protecting high-risk individuals and reducing the mortality and morbidity. Rapid development of COVID-19 vaccines has played a major role in drastically reducing COVID-19 Case Fatality Ratio (CFR). The mean cumulative Case Fatality Ratio (rCFR) of COVID-19 in the top-20 countries with vaccination rates was 1.18(95% CI: 0.73-1.62) on 5 Jan 2022 while the overall case fatality rate for Malaysia is 0.8% as of September 2022 [3,4].
Vaccines strategies included mRNA-based vaccines (Pfizer-BioNTech, Moderna), viral vector vaccines (Oxford-AstraZeneca, Johnson & Johnson), whole pathogen inactivated/killed vaccines (Sinopharm, SinoVac, Bharat BioTech) and subunit vaccines (Novavax) [5].
The efficacy and safety of a vaccine is evaluated in randomized clinical trials prior to approval and roll out of vaccines. Further, its effectiveness and side effects are monitored by observational studies, mainly case control and cohort studies in vaccinated population vs. unvaccinated who have clinical and laboratory confirmed COVID-19. Effectiveness in the real world can differ from the efficacy measured in a trial, because we can’t predict exactly how effective vaccination will be for a much bigger and more variable population getting vaccinated in more real life conditions [6].
Malaysia implemented community-wide COVID-19 vaccination using ChAdOx1 (AZD1222, Astra Zeneca), BNT162b2 mRNA (Pfizer BioNTech) and CoronaVac (Sinovac) vaccines in 20217 under the National COVID-19 Immunization Programme/ Program Imunisasi COVID-19 Kebangsaan. The efficacy and safety of these vaccines in a real-world community setting can be assessed from hospital data and research articles published from 2021 onwards. A systematic analysis of the available articles from various countries which used the three vaccines introduced in Malaysia was attempted. This helped to compare and analyse the effectiveness and safety of these vaccines in the real-world situation as well as get an idea of their effectiveness in our Malaysian population.
Materials and Methods
Search strategy
This was done as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) recommendations [8]. The electronic databases such Medline (via PubMed), EMBASE, Science Direct, SCOPUS and Google Scholar was systematically searched. Journal homepages and bibliographies were also searched to retrieve eligible studies. Manually, the articles were saved on a data extraction template created in Excel sheet and duplicated records were searched for reports [9].
Inclusion criteria
Study design: Observational studies (case-control and cohort studies) on effectiveness of the three vaccines in preventing hospitalisation or severe disease and side effects due to them.
Language: Articles published in the English language.
Publication date: From 2020 to 2022. Only full-text articles were included.
Setting: Worldwide.
Exclusion criteria
The exclusion criterion includes: Studies reporting exclusively on the immunogenicity of COVID-19 vaccine, Review articles, Abstracts, Ecological studies, Mathematical modelling analysis studies.
Study selection, extraction, and quality assessment
Full copies of articles identified by the search and meeting the inclusion criteria based on the title, abstract, and text contents are obtained for data synthesis. Three reviewers independently assessed studies for potential relevance, applied the eligibility criteria to the results. Duplicates were removed and any disagreements in the evaluation of studies were resolved through consensus among the authors.
Review synthesis
The systematic review approach was used to synthesize the evidence from the included studies. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline was followed to screen the studies (Figure 1).
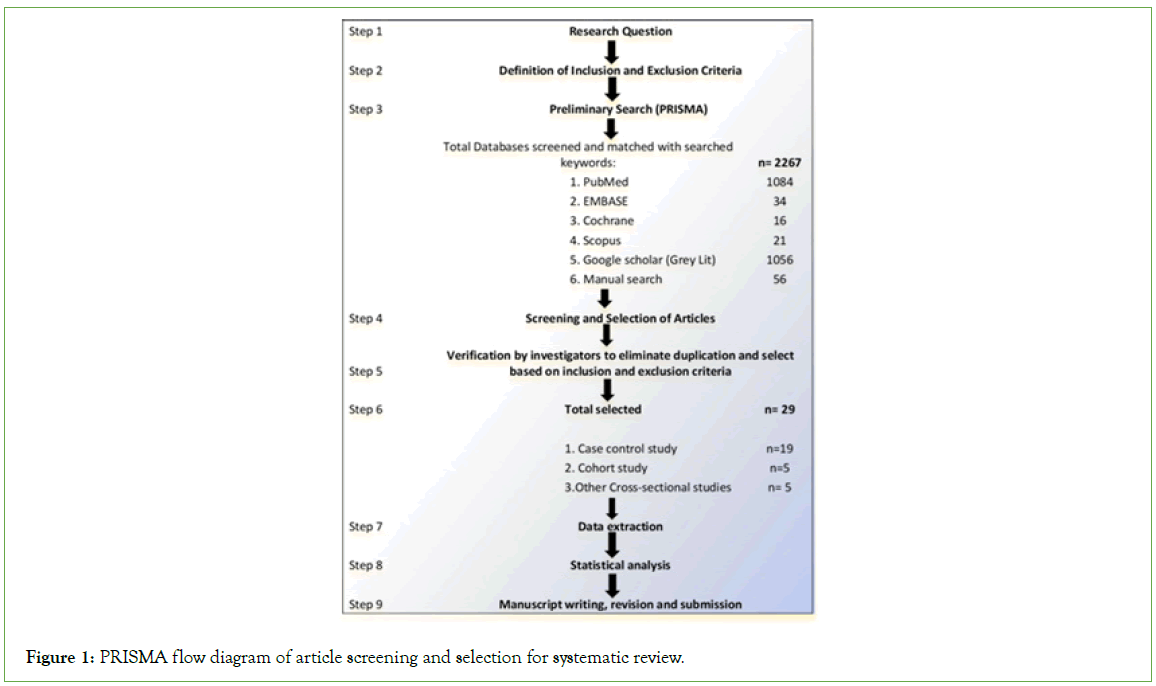
Figure 1: PRISMA flow diagram of article screening and selection for systematic review.
Studies where the patients had severe symptoms of COVID requiring hospital visits, hospitalisation or ICU admission and had already completed two doses of the vaccine were included. Cases were confirmed for COVID-19 infection by RT-PCR.
Results
A total of 29 real world effectiveness studies of the three vaccines namely BNT162b2 mRNA (Pfizer BioNTech), ChAdOx1 (AZD1222, Astra Zeneca) and CoronaVac (Sinovac) vaccine studies done worldwide were selected. These included 10 case-control studies each of BNT162b2 mRNA and CoronaVac vaccines while 9 studies were identified for ChAdOx1 meta-analysis [10-34]. These were compared against Malaysian data provided from the Programme Imunisasi COVID-19 Kebangsaan-PICK (National COVID-19 Immunisation Programme) which began from 24 February 2021 [34]. The Relative Risk Reduction (RRR) of the vaccines in preventing severe COVID and hospitalisation as well as ICU admission and death was calculated.
BNT162b2 produced an RRR ≥ 90% against severe symptomatic SARS-CoV-2 infections requiring hospitalization and/ or ICU admission (Table 1). The studies selected for ChAdOx1 vaccine effectiveness showed a wide range from 67.5%-95.6% (Table 2). Similarly for CoronaVac, the effectiveness ranged from 54%- 99.9% (Table 3). Meta-analysis of the data was further done using Microsoft Excel (Figures 2-5). p values were all significant and hence not displayed in the figures. A comparison on the effectiveness of the three vaccines was done to assess which vaccine elicited a better clinical outcome among the three (Table 4).
| BNT162b2 (Pfizer BioNTech) | |||
|---|---|---|---|
| Study | Effectiveness | Lower 95% CI | Upper 95% CI |
| Ref. Malaysia MOH | 90.3 | 88.8 | 91.6 |
| Samantha et al, US | 94.1 | 90 | 96 |
| Thiago Cerquerira et al, Brazil | 89.7 | 54.3 | 97.7 |
| Haas et al, Israel | 97.5 | 95 | 98.2 |
| Chung et al, Canada | 95.6 | 90.2 | 96.7 |
| Dagan et al, Israel | 92.5 | 75.2 | 100 |
| Flacco et al, Italy | 99.6 | 97.1 | 100 |
| Whitaker et al, UK | 94.7 | 86.1 | 97 |
| Stowe et al, UK | 95.2 | 86.9 | 98.1 |
| Abu Raddal et al, Qatar | 100 | 75 | 100 |
| Martinez-Bas et al, Spain | 94.7 | 60.4 | 99.3 |
Table 1: Comparative meta-analysis of BNT162b2 vaccine effectiveness against severe disease presentation.
| ChAdOx1(AZD1222, Astra Zeneca) | |||
|---|---|---|---|
| Study | Effectiveness | Lower 95% CI | Upper 95% CI |
| Ref. Malaysia MOH | 95.6 | 88.3 | 98.1 |
| Andrews et al, UK | 82.8 | 74.5 | 88.4 |
| Sheik et al, Scotland, UK | 90 | 83 | 94 |
| Thiago Cerqueira-Silva et al, Brazil | 89.9 | 83.5 | 93.8 |
| Thiruvengadam R et al, India | 81.5 | 9.9 | 99 |
| Sritipsukho P et al, Thailand | 83 | 70 | 90 |
| Hitchings et al Brazil | 86.2 | 77.3 | 94 |
| Stowe et al, UK | 92.5 | 75.1 | 97 |
| Whittaker et al, UK | 77.9 | 70.3 | 84.7 |
| Lopez Bernal2 et al, UK | 67.5 | 61.7 | 72 |
Table 2: Comparative meta-analysis of ChAdOx1 vaccine effectiveness against severe disease presentation.
| CoronaVac ( SinoVac) | |||
|---|---|---|---|
| Study | Effectiveness | Lower 95% CI | Upper 95% CI |
| Ref. Malaysia MOH | 72 | 66.9 | 73.9 |
| Premikha M et al, Singapore | 54 | 35 | 68 |
| Cerqueira-Silva T et al, Brazil | 81.3 | 75.3 | 85.8 |
| Suryatma A et al, Indonesia | 71.1 | 62.9 | 77.6 |
| Sritipsukho P et al, Thailand | 60 | 49 | 69 |
| Serrano-Coll et al, Columbia* | 99.9 | 87.8 | 100 |
| Ranzani et al, Brazil | 55 | 47.6 | 66.9 |
| Jara et al, Chile | 87.1 | 85 | 88.7 |
| McMenamin ME et al, HK | 91.7 | 88.7 | 94 |
| Yan VKC et al, HK | 58.9 | 50.3 | 66.1 |
| Copur et al, Turkey | 65 | 50 | 75 |
Table 3: Comparative meta-analysis of CoronaVac vaccine effectiveness against severe disease presentation.
| Mean | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|
| BNT162b2 (Pfizer BioNTech) | 94.9 | 81.7 | 97.7 |
| ChAdOx1 (AZD1222, Astra Zeneca) | 84.7 | 69.4 | 91.1 |
| CoronaVac (SinoVac) | 72.4 | 64.9 | 78.6 |
Table 4: Comparison of effectiveness of the three vaccines.
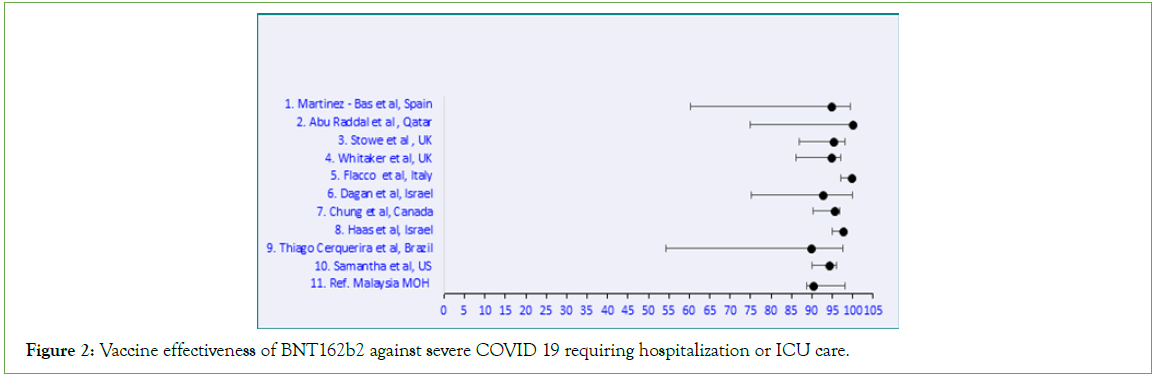
Figure 2: Vaccine effectiveness of BNT162b2 against severe COVID 19 requiring hospitalization or ICU care.
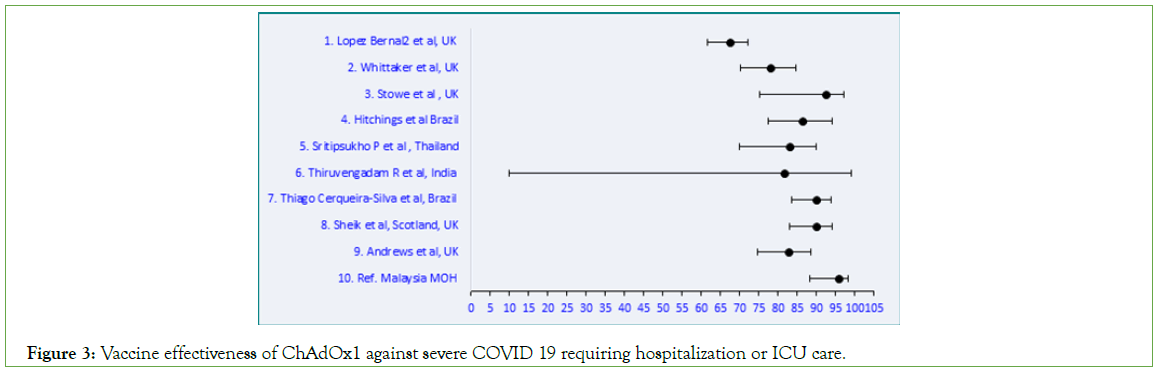
Figure 3: Vaccine effectiveness of ChAdOx1 against severe COVID 19 requiring hospitalization or ICU care.
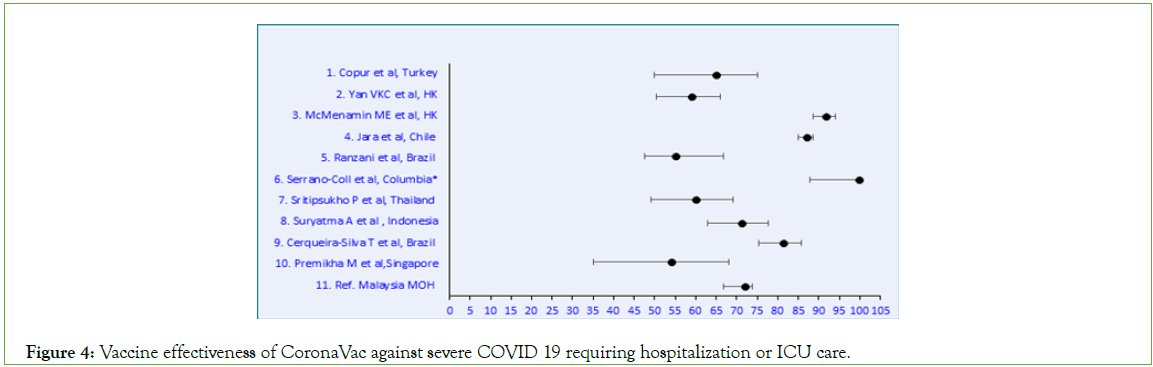
Figure 4: Vaccine effectiveness of CoronaVac against severe COVID 19 requiring hospitalization or ICU care.
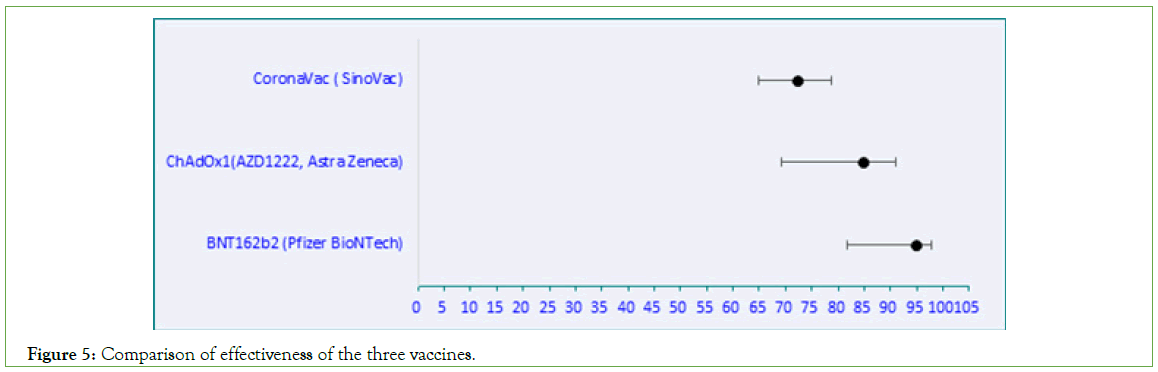
Figure 5: Comparison of effectiveness of the three vaccines.
Discussion
BNT162b2, ChAdOx1 and CoronaVac vaccines are the three vaccines which were introduced in Malaysia following Emergency Use Approval (EUA) by WHO after clinical trials in various countries. Once the program was rolled out, various observational studies were conducted in different parts of the world to assess its effectiveness. Our study was focused on assessing the effectiveness of the vaccines against severe COVID-19 including hospitalisation and ICU admission. Hence a systematic analysis was performed to obtain peer-reviewed articles on the effectiveness of these vaccines from different countries. A total of 29 studies were finally approved for our meta-analysis which included 10 for BNT162b2,9 for ChAdOx1 and 10 articles for CoronaVac.
Among the 10 studies selected for BNT162b2, the effectiveness was very high ( ≥ 90%) upon completion of two doses. WHO had approved Pfizer BioNTech vaccine against COVID-19 as studies had shown very high efficacy against severe disease and moderate efficacy against symptomatic SARS-CoV-2 infection [35]. BNT162b2 vaccine was the most administered vaccine in Malaysia compared to the other two vaccines (61.2%) [36]. The effectiveness of BNT162b2 vaccine is 90.3% which is within the range observed from other countries and WHO data.
Nine articles selected for ChAdOx1 vaccine showed a wider range in its effectiveness (67.5%-97.6%). The effectiveness of the vaccine reported from our country is the highest among the studies. The cohort receiving ChAdOx1 vaccine in Malaysia was 7.9% of the total population [36].
29.8% of Malaysian population had received CoronaVac (SinoVac) vaccine. The vaccine effectiveness of CoronaVac varied from 54%- 99.9% in the 10 studies selected for our meta-analysis. Malaysian data showed 72% effectiveness against severe disease. As per WHO, SinoVac-CoronaVac efficacy against hospitalization was 79% against severe COVID-19, and 100% against hospitalization starting 14 days after receiving the second dose [37]. A comparison between the three vaccines showed highest effectiveness for BNT162b2 (94.9%) followed by ChAdOx1 (84.7%) and SinoVac (72.4%).
Our study focused on the vaccine effectiveness in preventing severe disease, hospitalisation and ICU admissions. The effectiveness of these vaccines in preventing symptomatic disease or break-through infection cannot be assessed well and hence the overall effectiveness of these vaccines is not fully reflected as very few such studies are available.
Vaccine Efficacies (VE) ranging from 50% to 95% against symptomatic COVID-19 infections have been reported, using varying endpoint definitions. Real-world evidence from vaccine- rollout programs has shown that COVID-19 vaccines are highly effective against severe disease, hospitalization, and death, and reduce both asymptomatic infection and within household transmission [38]. According to Food and Drug Administration in order for an experimental COVID-19 vaccine to get the green light, it would need to be safe and “prevent disease or decrease its severity in at least 50% of people who are vaccinated” [39]. The 50% efficacy threshold set for COVID-19 vaccines is because COVID-19 was deemed such a severe disease, that if a vaccine is only 50% effective, it’s still worth using. Fortunately, the emerging data on COVID-19 vaccines suggests that the vaccines are very safe with high efficacy, at least against some of the variants. AstraZeneca vaccine seems to have lower efficacy than the Pfizer vaccine at preventing mild to moderate disease, but has proven very useful in preventing severe disease, with nearly 100% efficacy against at least some variants of the virus that cause COVID-19. Given the very real risks from COVID-19 and the evidence that COVID-19 vaccines provide protection, especially against severe disease, hospitalization and death; makes it a good case to actively promote immunization with the currently available vaccines which have been shown to be very effective.
Conclusion
The wealth of real-world evidence confirms the remarkable efficacy of COVID-19 vaccines in thwarting severe disease, hospitalization, and mortality. Our study serves as a robust validation of the critical necessity to continually evaluate the effectiveness of these vaccines in safeguarding against severe COVID-19 cases. As we move forward, this knowledge remains pivotal in enhancing public health strategies and combating the pandemic effectively.
Limitations of the Study
• Multiple variants of COVID-19 have shown different severity in presentation, especially more with delta variant.
• Age related response is varied with severe symptoms and hospitalisation in aged population. Some studies have stratified the samples age wise to emphasize the skewing with people >60 years being more affected.
• Variation in country-wise data may reflect differences in the roll out of the vaccines with one or two of the three vaccines being used less, rolled out later, and phased out or regional factors.
• Asymptomatic COVID-19 accounts for a large percentage of the clinical presentation and hence the effectiveness of the vaccine on this group cannot be assessed as they don’t subject them to testing or visit clinics and hence are not captured in many studies [40].
• Booster dose effect on immune response need to be studied further.
• The pandemic is still running its course with newer virus variants emerging and the effectiveness of vaccine and boosters need to be further assessed.
• Better understanding of the disease pathology, newer antiviral drugs and immunotherapy has helped to improve the management of severe COVID-19 which also affects the final outcome of these studies, especially those done in late 2021 and 2022.
• Studies on the vaccine effectiveness on pregnant women, immunocompromised and those below 18 years of age are few.
Suggestions
• Subgroup analysis (based on age groups) will help to understand the age group most affected and whether it’s affecting the overall data.
• Comparison between RCT and observational studies would give a more accurate picture of the difference between efficacy and real-world effectiveness.
Declarations
Ethics approval statement
The study was approved by the Institutional Ethics Board of the university.
Competing interests
There are no known competing financial interests or personal relationships that could have influenced our work.
Author contributions
Anita Devi Krishnan Thantry: Conceptualization, methodology and supervision of the project, Original draft preparation; Palaniappan A/L Palaniappan Lakshmanan, Edwin Cheah Shenq and Dhanraaj A/L Kunasakaran: Data collection and analysis, Design of figures and tables.
Funding
This study was part of a student project for which no funding was involved.
Availability of data and materials
All data supporting the findings of this study are available within the paper and its supplementary information.
References
- World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report-94. 2020:61-66.
- WHO Coronavirus (COVID-19) Dashboard. 2020.
- Haider N, Hasan MN, Khan RA. The Global case-fatality rate of COVID-19 has been declining disproportionately between top vaccinated countries and the rest of the world. IJID Regions. 2023;6:159-166.
[Crossref] [Google Scholar] [PubMed]
- Ministry of Health, Malaysia. COVID-19 Deaths in Malaysia. 2022.
- Kyriakidis NC, Lopez-Cortes A, Gonzalez EV. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6(1):28.
[Crossref] [Google Scholar] [PubMed]
- WHO: Vaccine efficacy, effectiveness and protection. July 2021.
- Jayasundara P, Peariasamy KM, Law KB. Sustaining effective COVID-19 control in Malaysia through large-scale vaccination. Epidemics. 2021;37:100517.
[Crossref] [Google Scholar] [PubMed]
- Moher D, Shamseer L, PRISMA-P group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. 2015;4(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Girma D, Dejene H, Adugna L. COVID-19 case fatality rate and factors contributing to mortality in Ethiopia: A systematic review of current evidence. Infect Drug Resist. 2022;15:3491-3501.
[Crossref] [Google Scholar] [PubMed]
- Martínez-Baz, Miqueleiz A, Working Group for the Study of COVID-19 in Navarra. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Euro surveill. 2021;26(21):2100438.
[Crossref] [Google Scholar] [PubMed]
- Abu-Raddad LJ, Chemaitelly H, National Study Group for COVID-19 Vaccination. Waning mRNA-1273 vaccine effectiveness against SARS-CoV-2 infection in Qatar. N Engl J Med. 2022;386(11):1091-1093.
[Crossref] [Google Scholar] [PubMed]
- Stowe J, Andrews N, Kirsebom F. Effectiveness of COVID-19 vaccines against Omicron and Delta hospitalisation: Test negative case-control study. MedRxiv. 2022; 30;13(1):5736.
[Crossref] [Google Scholar] [PubMed]
- Whitaker HJ, Tsang RSM, Byford R. Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response amongst individuals in clinical risk groups. J Infect. 2022;84(5):675-683.
[Crossref] [Google Scholar] [PubMed]
- Flacco ME, Soldato G, Martellucci CA. Interim estimates of COVID-19 vaccine effectiveness in a mass vaccination setting: Data from an Italian province. Vaccines. 2021;9(6):628.
[Crossref] [Google Scholar] [PubMed]
- Dagan N, Barda N, Kepten E. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412-1423.
[Crossref] [Google Scholar] [PubMed]
- Chung H, He S, Nasreen S. Effectiveness of BNT162b2 and mRNA-1273 Covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada: Test negative design study. BMJ. 2021;20(374):1943.
[Crossref] [Google Scholar] [PubMed]
- Haas EJ, Angulo FJ, McLaughlin JM. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalizations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet. 2021;397(10287):1819-1829.
[Crossref] [Google Scholar] [PubMed]
- Cerqueira-Silva T, Katikireddi SV, Oliveira VA. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nat Med. 2022;28(4):838-843.
[Crossref] [Google Scholar] [PubMed]
- Carlson SJ, Tomkinson S, Blyth CC. COVID-19 vaccine knowledge, attitudes, and experiences of health care workers in Perth, Western Australia: A qualitative study. PLoS One. 2022;17(12):279557.
[Crossref] [Google Scholar] [PubMed]
- Bernal JL, Andrews N, Gower C. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ. 2021;373:1088.
[Crossref] [Google Scholar] [PubMed]
- Hitchings MDT, Ranzani OT, Dorion M Effectiveness of ChAdOx1 vaccine in older adults during SARS-CoV-2 Gamma variant circulation in São Paulo. Nat Commun. 2021;12(1):6220.
[Crossref] [Google Scholar] [PubMed]
- Sritipsukho P, Khawcharoenporn T, Siribumrungwong B. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the delta variant-dominant pandemic: A test-negative case-control study. Emerg Microbes Infect. 2022;11(1):585-592.
[Crossref] [Google Scholar] [PubMed]
- Thiruvengadam R, Awasthi A, Medigeshi G. Effectiveness of ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection during the delta (B.1.617.2) variant surge in India: A test-negative, case-control study and a mechanistic study of post-vaccination immune responses. Lancet Infect Dis. 2022;22(4):473-482.
[Crossref] [Google Scholar] [PubMed]
- Sheikh A, McMenamin J, Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021; 397(10293):2461-2462.
[Crossref] [Google Scholar] [PubMed]
- Andrews N, Stowe J, Kirsebom. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532-1546.
[Crossref] [Google Scholar] [PubMed]
- Copur B, Surme S, Sayili U. Effectiveness of CoronaVac vaccination against COVID-19 development in healthcare workers: Real-life data. Future Microbiol. 2022;17(17):1381-1391.
[Crossref] [Google Scholar] [PubMed]
- Yan VKC, Wan EYF, Ye X. Effectiveness of BNT162b2 and CoronaVac vaccinations against mortality and severe complications after SARS-CoV-2 Omicron BA.2 infection: A case-control study. Emerg Microbes Infect. 2022;11(1):2304-2314.
[Crossref] [Google Scholar] [PubMed]
- McMenamin ME, Nealon J, Lin Y. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22(10):1435-1443.
[Crossref] [Google Scholar] [PubMed]
- Jara A, Undurraga EA, González C. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875-884.
[Crossref] [Google Scholar] [PubMed]
- Ranzani OT, Hitchings MDT, Murilo Dorion M. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of Covid-19 in Brazil: Test negative case-control study. BMJ. 2021;374:2015.
[Crossref] [Google Scholar] [PubMed]
- Serrano-Coll H, Miller H, Guzmán C. Effectiveness of the CoronaVac® vaccine in a region of the Colombian amazon, was herd immunity achieved? Tropical diseases. Trop Dis Travel Med Vaccines. 2022;8(1):2.
[Crossref] [Google Scholar] [PubMed]
- Suryatma A, Anasi R, Hananto M. Real world performance of inactivated SARS-CoV-2 vaccine (CoronaVac) against infection, hospitalization and death due to COVID-19 in adult population in Indonesia. MedRxiv. 2022.
- Premikha M, Chiew CJ, Wei WE. Comparative Effectiveness of mRNA and inactivated whole-virus vaccines against coronavirus disease 2019 infection and severe disease in Singapore. Clin Infect Dis. 2022;75(8):1442-1445.
[Crossref] [Google Scholar] [PubMed]
- Suah JL, Keng Tok PS, Ong SM. PICK-ing Malaysia’s epidemic apart: Effectiveness of a diverse COVID-19 vaccine portfolio. Vaccines (Basel). 2021;9(12):1381.
[Crossref] [Google Scholar] [PubMed]
- WHO Strategic Advisory Group of Experts on Immunization (SAGE). The Oxford/AstraZeneca (ChAdOx1-S [recombinant] vaccine) COVID-19 vaccine: what you need to know. 2022.
- Ministry of Health Malaysia .Vaccinations in Malaysia-COVIDNOW. 2022.
- WHO Strategic Advisory Group of Experts (SAGE). The sinopharm COVID-19 vaccine: What you need to know. 2022.
- Feng S, Philips DJ, White. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032-2040.
[Crossref] [Google Scholar] [PubMed]
- FDA. Coronavirus (COVID-19) update: FDA takes action to help facilitate timely development of safe, effective COVID-19 vaccines. 2020.
- Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: A narrative review. Ann Intern Med. 2020;173(5):362-367.
[Crossref] [Google Scholar] [PubMed]
Citation: Thantry ADK, Palaniappan, Shenq EC, Dhanraaj (2023) Effectiveness of SARS-CoV-2 Vaccines used in Malaysia: A Systematic Review and Meta-Analysis. J Vaccines Vaccin. S23:001.
Copyright: © 2023 Thantry ADK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

