Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2020) Volume 11, Issue 5
Effective SARS-CoV-2 Vaccines on the Horizon
Soma Ghosh1 and Prasanta Kumar Ghosh2*2Department of Biotechnology, Ministry of Science and Technology, Government of India, New Delhi-110058, India
Received: 12-Aug-2020 Published: 03-Sep-2020, DOI: 10.35248/2157-7560.20.11.428
Abstract
Novel coronavirus identified as SARS-CoV-2 has brought unprecedented sufferings to people in almost all countries across the globe during the recent time. Efforts are continued to develop effective vaccines against the disease. Presently, the efforts of six groups namely, University of Oxford, Oxford, UK; Sinovac, China; Moderna Inc., USA; Wuhan Institute, China; Beijing Institute, China; and Bio N Tech, Germany are more significant. Noteworthy progress in the development of vaccines is in sight, and by the mid-2021 new vaccines may start appearing in the market. All these six vaccines are deployed through intramuscular route. There are a few others which are in the developmental stage and are to be delivered through intradermal, intranasal and event through the oral route. Specialized adjuvants have been used to elicit stronger immune response in certain types of candidates. Sensitized immune cells and genetically modified cells are also being experimented upon to contain the virus through immunological mode.
Keywords
SARS-CoV-2; COVID-19; TMPRSS2; ACE2 receptor; Corona; Vaccines
Introduction
Coronavirus disease 2019(COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported for the first time from China [1] during December 2019 through Dr.Li Wenliang, a medical doctor (and a whistle blower), who was working in a Wuhan hospital and who sent a message through “We chat” to his friend about the disease. Prior to this incident, the group of viruses designated as severe acute respiratory syndrome coronavirus (SARS-CoV-2) were described in the literature earlier, and for the first time the Chinese scientists in 2002 described new isolates that were found in Guangdong province of southern [2] China ,where the first infected human was identified and described. The infection from the coronavirus SARS-CoV-2 turned out to be pandemic soon after December 2019. Presently, the whole world is treating a mega number of infected individuals [3] suffering from COVID-19 caused by the novel coronavirus.
The purpose of this paper is to assess the horizon of fast appearance of safe and effective vaccines against the disease.
Methodology
The status of progress on the development of effective vaccines has been collated from published information. The sources of information used have been cited. The information base has been critically evaluated incorporating observations and comments.
Coronavirus: SARS-Co V-2 invasion of human body
Coronavirus has crown-like spikes on its outer surface. The virus is essentially a spheroid in structure with spikes protruding outwards on its body surface. Coronaviruses are presently known in twenty six species, which have been divided into four genera, classified based on the antigenic cross-reactivity and genetic makeup; the four genera are designated as alpha, beta, gamma and delta. The corona viral strains pathogenic to human include the genera alpha and beta.SARS-CoV-2 belongs to the genera ‘beta’ and is a positive-sense single-stranded RNA virus [4-6].
SARS-CoV-2was first recorded on 12th December 2019 at Wuhan China [7]. The investigators had found that the SARS-CoV- 2 isolated from the bronchoalveolar lavage fluid of a critically ill patient in China could be neutralized by convalescent sera obtained from several patients, implying thereby that all the viruses from different patients were the same .The genome sequences of these novel coronaviruses matched nearly 96% to the bat-coronavirus genome and about 79.6% of other known coronaviruses, described earlier in the literature. It was reaffirmed that the SARS-CoV-2 virus uses the same host -cell entry port namely the angiotensin converting enzyme 2 (ACE2) receptor proteins, present on various host cells, to enter the host bodyas other severe acute respiratory syndrome coronavirus (SARS-Co V) virus does. The complete nucleotide sequence of the genome of SARS-CoV-2 with 29,903 nucleotides was disclosed [8,9] by China in January 2020.
SARS-CoV-2 had been causing severe respiratory diseases along with the Middle East Respiratory Syndrome (MERS) coronavirus intermittently from time to time during the last two decades and currently another closer relative, theSARS-CoV-2 has caused the present pandemic. The genomic sequence of all these viruses is similar to a great extent (50-80%). The entry point of human host receptor cells are the ACE2 receptor proteins, expressed by the host cells. The host cell receptors ACE2 were identified [10,11] as the entry-gate receptors for the whole range of SARS-CoV-2 viruses.
Comprehension of how SARS-CoV-2 enters human cells is important in deciphering and curving the spread of the disease. All SARS-CoV-2 viruses have on their outer surface the spike proteins (S) which mediate their entry into host human cells. Activation of (S) is essential for SARS-CoV-2 infectivity. For this to happen, the spike protein first binds to the host cell ACE2 through its receptor-binding domain (RBD). This binding is proteolytically activated by the adjacent host cell proteases. The activated and cleaved action results in the generation of an N-terminal surface unit S1 which binds to the receptor and the C-terminus Trans membrane unit which is for cell membrane fusion. Actions of S1 and S2 together trigger receptor-mediated endocytosis, transporting the viron particles into the host cell endosomes. The expression and action of the trans membrane protease serine 2(TMPRSS2) of the type II trans membrane serine protease (TTSP) family is responsible for cleaving the (S) protein of SARS-CoV-2, which follows virus fusion with the target host cells. Activation pathways of the spike protein by other TTSPs such as the human airway trypsin-like protease (HAT) are different from the TMPRSS2 activation pathway [12, 13]. It has been found that SARS-CoV-2 RBD has higher ACE2 binding affinity than SARS-CoV-2 RBD, which implies that SARS-Co V-2 is more infectious than the SARS-CoV-2 [14].
In a pictorial diagram, the viral infection process and the budding out of young viral particles from the infected cells have been presents at Figure 1.
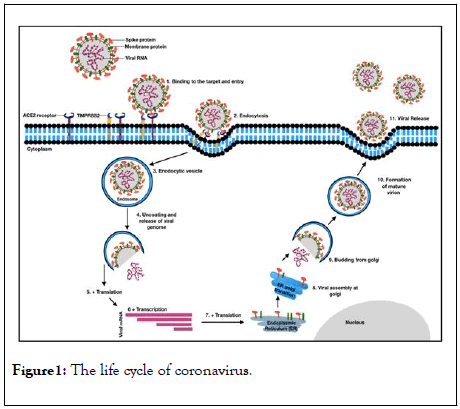
Figure 1: The life cycle of coronavirus.
The spike (S) protein of SARS-CoV-2 binds to the ACE 2 receptor of the host cell (Step-1) at the receptor binding domain. Binding of the ACE2 followed by priming by the adjacent serine protease TMPRSS2 cleaves the trimeric S-protein, activating and causing conformational changes and leading to the endocytosis of the virus and these activities result in the virus to enter the host cell (Steps 2-3). After endocytosis the viral envelope is peeled off, which leads to genomic RNA to be present in the cytoplasm of the host cell (Step-4).Viral RNA is translated by the host translational machinery. Following replication and subgenomic RNA synthesis, the viral structural proteins, spike (S), envelope (E) and membrane (M) are translated and inserted into the endoplasmic reticulum (ER) (Steps 5-7). These proteins move along the secretary pathway into the membranes of endoplasmic reticulum – Golgi intermediate compartment (ERGIC) (Step-8). Virus goes inside the Golgi vesicle to form the mature virion (Steps 9-10). Finally, the virion-containing vesicle fuses with the membrane of the host cell to release the virus (Step-11).
Results and Discussion
Aim and goal for SARS-CoV-2vaccines development
Exposure to multiple epidemics from H1N1 influenza, Nipah, Ebola, Zika, SARS-Co V, MERS during the last two decades had provided enormous opportunities for the scientists to understand the basis of this illness, which enlarged scientific knowledge to tackle such calamities. As a result, different kinds of vaccine technologies have evolved. These include the inactivated viral particles suspended on an adjuvant; the use of attenuated live viral particles; using of the nucleic acid substances such as the designed DNA molecules in plasmid carriers; engineered RNA molecules that code for antigenic proteins; and the use of replicating or non-replicating viral vectors genetically engineered, to express immunogenic proteins of the disease-causing viruses. In these ways the immune machinery was primed. These multiple strategies enabled assessment of efficacy namely, whether the technology produces enough neutralizing immune-protective immunoglobulin (Ig) molecules, particularly Ig G to neutralize the viral particles and /or whether the recipients are producing enough cytotoxic CD8+ T cells to destroy the virus – infected cells and engulf the virus particles along with the macrophages and other activated lymphocytes.
In two pictorial cartoons, at Figures 2 and 3, the major steps and aim in the acquired immunity development against an antigen are depicted.
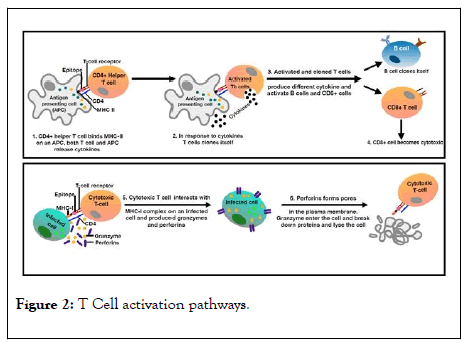
Figure 2: T Cell activation pathways.
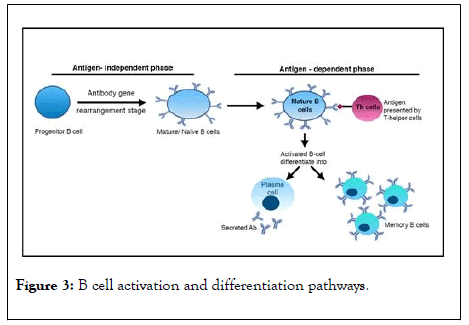
Figure 3: B cell activation and differentiation pathways.
When a mature B cell encounters antigens presented by T helper cells that bind to its B cell receptor, it becomes activated. It then proliferates and differentiates into plasma B cells and memory B cells with a high-affinity for the original antigen. The memory cells are long-lived and the plasma cells may secrete antibody for weeks after the initial infection.
Safety features
All the vaccines being developed are to be safe on all the features of safety. Licensed vaccines should not compromise on any kinds of significant safety issues particularly to minimize the risk of antibody-dependent enhancement (ADE) causing enhanced viral infection or eosinophilia. Adversities have been encountered by the researchers [15] in the past while developing SARS-CoV-2 vaccines. The risks from ADE while developing viral vaccines are yet inadequately understood. Generally, it has been found that this phenomenon is related with the hyperactivity of Th-2 pathway while the immune system reacts to the viral antigens. Some correlates in terms of measurements of Ig G subclass and certain cytokine expression profiling, especially IL-6 are thought to be indicative of Th-2 hyperactivity. During human trials, adequate safety observations are to be made so as to statistically show that the risks of ADE are minimal. The first aspect of vaccine development would be to ensure safety and the second aspect is that it should be effective. Effectively implies that these need to have features of adequate immunogenicity, stability, and manifestation of long-lasting immunity.
Establishing the safety features of SARS-CoV-2 vaccines require generation of information in appropriate animal model. Information generation may also include long term data on DNA integration if any, into the host genome where replicating, genetically modified DNA viral vectors are used. Safety studies have been made in different animal model such as mice, rats, rabbits, guinea pigs and macaques. Initial studies are often carried out in Balb/c or C57BL/6 mice.
In one study [16] four candidate vaccines for humans with or without alum adjuvant were evaluated in Balb/c andC57BL/ 6mice. Interestingly, all the four vaccines tested induced serum neutralizing antibody in proportion to dosage increase. Significant reductions of live SARS-CoV-2 virus two days after challenge were observed for all vaccines. However, it was observed that all mice exhibited histopathologic changes in lungs two days after challenge including all animals vaccinated or given live virus or influenza vaccine, or phosphate-buffered saline. These results suggested that infection had occurred in all animals again before sacrifice. Histopathology studies in animals given one of the SARS-CoV-2 vaccines were uniformly a Th2- type immunopathology with prominent eosinophil infiltration. It was concluded that while the vaccines tested induced cloned antibody production and protection against infection with SARS-CoV-2,the factor of elevation in the chances of occurrence of Th2-type immunopathology needs to be kept in mind as the resulting hypersensitivity of the vaccines to SARS-CoV-2 component scan lead to multiple adverse manifestations, making the vaccine useless for use in human. The main limitations of the study were that the animals used were not humanized animals and therefore these results are anticipated to differ from the manifestations in human subjects, although much insight could be had from these experiments.
In another study ,whole UV-inactivated SARS-CoV-2 particles, bearing multiple epitopes and proteins, was used as a candidate vaccine and the efficacy was being studied in 6-month-old adult BALB/c mouse model; it was observed that while immunization of adult mice with or without alum, resulted in partial protection from lethal dose challenge against live SARSCoV- 2 particles, the vaccine induced excessive eosinophilic infiltration in mouse lungs [17], resulting from Nucleocapsid proteins of the inactivated SARS virus. Such adverse effects need to be especially taken note of while evaluating the candidate SAR-CoV-2 vaccines as there are considerable similarity in proteins of SARS-CoV-2 and SARS-CoV-2.
In viral vaccine development, while the aim is to prime the immune system in the Th-2 pathway in a balanced manner to produce clonal immunoglobulin ’ s, it is almost impossible to precisely develop only one set of target immunoglobulin’s. There would be opportunities for the immune system to also produce adequate quantities of non-target-epitope-specific immunoglobulins. Such immunoglobulin’s would bind to the viruses but would not elicit adequate opsonization process, being smaller in concentrations. Human body has cells which do not express the vial receptors ACE2; however, such cells may have Fc receptors which can bind to one end of the non-target neutralizing antibodies and such binding can facilitate the viral particles to bind to the other end. It has been theorized that by such a process the viral particles can be closer to the non-specific immune cells or even other types of cells and thereafter interaction of the cellular proteases would cleave the viral docking proteins like the S proteins for SARS-CoV-2 and would enable the virus to enter the cells through endocytosis and would multiply inside such cells. Increased production ofa large number of infective viruses such as Dengue virus, Yellow fever virus, Zika virus, HIV and corona viruses have been found in multiple sets of experiments with animals as also in practice in human when certain vaccines were used such as the Dengue vaccine. The safety issues shall therefore require [18-20] exhaustive assessment when new SARS-CoV-2 vaccines are developed.
In a recently held virtual meeting, held under the umbrella of the International Coalition of Medicines Regulatory Authorities (ICMRA), the World Health Organisation (WHO), the European Commission and others from 17 countries held on 18th March 2020, the guidelines [21] for the generation of preclinical data required to support first in human clinical trials were discussed. It was agreed that because of exigency of urgency for developing a vaccine against SARS-CoV-2, the toxicity data generation in animals need not be conducted prior to first-inhuman clinical trials for well-characterized investigational vaccines. Further, it is not required to demonstrate the efficacy in animal challenge models prior to proceeding for clinical trials. While generating data using healthy young adult volunteers, in Phase I and II studies, the volunteers are to be informed about any potential risks and their health would have to be monitored closely for toxicity and adverse effects if any, after dosing them. Only after adequately being satisfied about the safety in Phase I studies, larger number of volunteers need be dosed in Phase II and Phase III trials. While there are ACE2 transgenic animals [22-24],the ICMRA had not insisted on generating information of live – virus challenging experiments in containment facilities( Biosafety-level3), using such transgenic animals. Clearly therefore, the Clinical Trials in various phases of human exposure of the candidate vaccines would assume increased rigor from safety assessment.
“Operation Warp Speed” for COVID-19 vaccine development: USA
The US Government through its “ Operation Warp Speed ” COVID-19 vaccine program had earlier supported five SARS-CoV- 2 vaccine development programs on a fast tract mode. These projects include Modern’s m RNA-1273 vaccine; BioN Tech/Fosun Pharma /Pfizer ’ s BNT 162a1, BNT 162b1, BNT162b2and BNT 162c2 m RNA vaccines; MSD and IAVI initiatives based on VSV vector based-vaccine; JandJ’ s replication-defective adenovirus-26 vector based vaccine; and Astra Zeneca and University of Oxford’s replication defective adenovirus vector based vaccine namely Ch AdOx1 nCoV-19 vaccine [25]. Another biotech company Novavax, Maryland, USA had received [26] a support of US $1.6 billion contract from the US Government for developing a COVID-19 vaccine through the above program. In total therefore, as of July 11, 2020 six companies had received US Government ’ s support under the program to develop SARS-Co V-2on a fast mode. MSD has not yet been able to take steps [27] towards the development of a SARS-Co-v-2 vaccine. In a Business wire news on May 26, 2020 it was revealed [28] that the International AIDS Vaccine Initiative (IAVI) , USA and MSD entered into a collaborative tie upto developed vaccine against SARS-CoV-2 using the recombinant VSV (r VSV) vaccine platform. MSD has been working on the on RSV backbone and had developed their Ebola virus vaccine (Ebola Zaire Vaccine, live-ERVEBO) earlier.
Types of SARS-CoV-2 candidate vaccines
Several investigators all over the world are engaged in the development of an effective vaccine againstSARS-CoV-2. The World Health Organization (WHO) had published [29] on 31st July2020 the list of experimental vaccines being pursued all over the world. This list had details about the types of candidate vaccines, the developer institutes/ companies and the target structure of SARS-CoV-2 attacked for the intervention of the virus. Certain other publications [30-33] also had details on the global race on development of SARS-CoV-2 vaccines. Even though the total numbers of candidate SARS-CoV-2 initiatives from these publications are199 in total, in this paper the information on 169 were compiled and integrated; these include the information provided in the WHO list and a few more. The kinds of vaccines which are being developed as of 31st July 2020 is depicted in Figure 4.
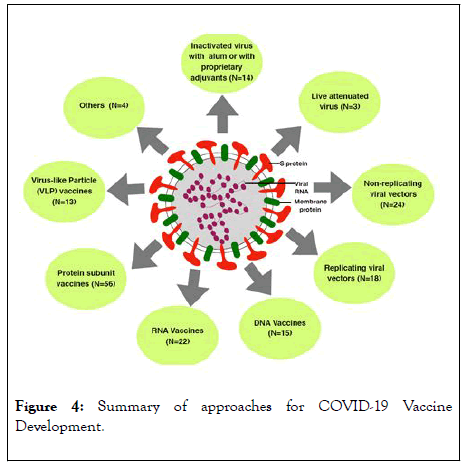
Figure 4: Summary of approaches for COVID-19 Vaccine Development.
An overview of the different vaccine types currently in clinical development. Nine different technology platforms are under research and development as of July, 2020 to create an effective vaccine against COVID-19. N=Number of candidates in each technology platform. A total of 169 candidates depicted.
All the169 phenotype candidates target SARS-Co V-2 virus neutralization and destruction. One of these is to upgrade innate immunity in the recipient. The WHO list depicts 26 numbers of these which are in clinical evaluation stage. In total27 companies and institutions had made considerable progress as of 31st July 2020 and26 numbers had reached Phase I clinical trial stage and further. Among the candidate vaccines in preclinical stage or even in the late conceptual stage there are 143 numbers. These include 139 cases of the WHO list. In case of Tulane University, USA [34], which is included in the list of 143, it was revealed that the University had received US$1 million assistance and would develop a SARS-CoV-2 vaccine.
Application mode of SARS-Co V-2 candidate vaccines
Most of the candidate vaccines depicted in Figure 4 are to be deployed as a sterile intramuscular injection. A novel route of intradermal injection of a DNA construct followed by electroporation (Inovio, USA) has also been described [35]. A delivery of SARS-CoV-2 spike protein antigens through skin [36] using micro-needle patches was developed and tested in animal model by the University Of Pittsburgh School Of Medicine. The vaccine developed antibodies specific to the virus in mouse model. A small number [37,38] of companies (Autiimmune, USA and Intravacc, Netherland) are also developing vaccines which are to be deployed through intranasal route. Oral route of administration has been deployed in one study where genetically modified probiotic Bifid bacterium lingum coding for spike glycoprotein of SARS-CoV-2 has been [39] used (Symvivo Canada).The candidate product is called as bac TRL-Spike Vaccine .Vaxare, USA announced [40] that it was in the process of manufacturing its oral SARS-CoV-2 recombinant constructs in bulk in the cGMP facilities of Kindred Bio, USA. The bulk vaccines were to be evaluated for efficacy and safety. The company had earlier developed other oral vaccines against influenza. The efficacy of the oral SARS-CoV-2 vaccine in lab animals was already tested and found to be useful. Immunitor LLC, Canada had developed a thermostable therapeutic vaccine formulation in the form of oral tablets, manufactured from pooled plasma of COVID-19 patients and heat-inactivated the product to ensure full killing of any viral particle. The recipients would have to take one tablet a day for a month to elicit efficacy. The formulation has been under evaluation by the USFDA on volunteers for 15 days when baseline and post-treatment standard safety parameters would be compared [41]. Lentiviral vector engineered to express SARS-CoV-2 antigens has been used to modify the dendritic cells (LV-DC). The LV-DC has been used to activate CTLs directed to SARS-CoV-2 virus. In this approach artificial antigen presenting cells are then infused into the subjects who would receive total 5 × 106 cells of LV-DC and 1x108 antigen-specific CTLs via sub-cutaneous injection and intravenous infusion, respectively and evaluated over a period [42] to find the efficacy (Shenzhen Geno-Immune Medical Institute, China). Aivita Biomedical, USA has developed its patient-specific vaccine platform [43] where the recipient’s own immune dendritic cells are picked up from peripheral blood monocytes-derived dendritic cells, purified and loaded ex-vivo with multiple SARS-CoV-2 spike protein antigens which are associated with binding and infectivity. The antigen-loaded and sensitized dendritic cells are re-administered to the patient through a subcutaneous injection, with the aim of creating adaptive immunity. The company had used the technology platform to treat other diseases including certain cancer. The technology of creating adaptive immunity against the SARS-CoV- 2 virus is presently under Phase Ib-II Trial [44] by the USFDA.
Use of proprietary adjuvants in certain candidates is anticipated to induce more CD4+ and CD8+ T-cell to produce more T and B-cell memory responses and may boost clonal antibody concentrations more than when alum is used. Live attenuated virus vaccines may continue to continuously present the antigens to the immune cells and therefore may not require additional special adjuvants in the formulations.
Two other approaches are somewhat different with more novelty. One is from Velo Therapeutics Ltd., UK, an immunooncology company developing tumor antigen-coated oncolytic viruses as therapeutic vaccines propose to use [45] its PeptiCRAd technology to coat an adenovirus vaccine vector which is engineered to express coronavirus associated spike proteins, with HLA-matched peptides optimized to further boost CD8+ T-cell immune responses. The PeptiCRAd (Peptide-coated Conditionally Replicating Adenovirus) technology [46] is another innovative way of combining two clinically proven cancer immunotherapy approaches namely, an oncolytic Adenovirus and a peptide vaccine; by such combination, it takes advantage of the best features of both technologies. Using this technology platform, the company anticipates eliciting better immune responses. Velo Therapeutics obtained the license for using the technology from the University of Helsinki [47]. The other approach is from VBI, USA which uses its novel virus-like particle (VLP) vaccine technologies. VLPs are self-assembled viral protein complexes, spherical in shape with sizes ranging 20–800 nm. The VLPs used in the production platform can be generated through r DNA technology in bacteria, yeast, insect cells, plants and mammalian cells [48]. VBI, uses it’s enveloped VLPs (eVLPs) produced [49] in mammalian cells, thereby having components of host cell derived substances which have closer resemblance with human cells and are anticipated to be more efficiently presented to the immune cells, thereby eliciting more immunogenicity of the target antigens. Using this platform technology, the company is developing multiple other candidate vaccines against viral diseases such as CMV, GBM and ZIKA. VBI is developing SARS-CoV-2 vaccine candidate in collaboration with the National Research Council of Canada.
Candidate vaccines in clinical trials
Once the science and the lab-based techniques are welldeveloped, the candidate vaccines can be put to scientific evaluation. Large-scale manufacture and distribution are the downstream issues which also need to be tackled with greater vigor to make a project successful. One of the biggest challenges of manufacturing the vaccine in large scale is the construction and validation of production platforms which are capable of making the vaccine on a large scale. Once the manufacturing challenge is sorted out, there has to be a well-neat distributional channel so as to make the availability of the vaccine to the eligible recipients. Only when all the challenges are successfully met, the project becomes successful. The following provides a gist of how the main developers of the vaccine are gearing up. All these candidates are in clinical evaluation stages.
Figure 5 provides the summary of the 27 vaccine candidates which are in clinical evaluation stage. The following are additional details about these candidates.
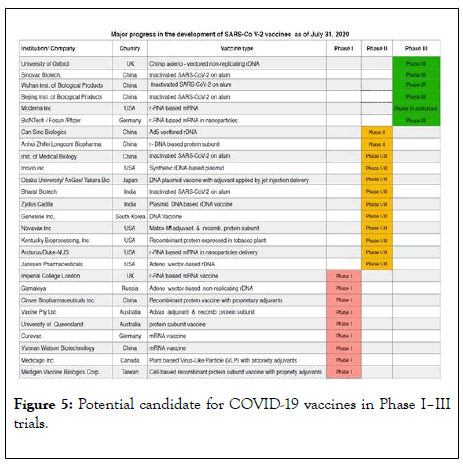
Figure 5: Potential candidate for COVID-19 vaccines in Phase I–III trials.
Twenty seven major institutions/ companies are conducting clinical trials and are in different phases of clinical development. Phase I stage is shown in Red; Phase II is in Orange; Phase III is in Green.
Phase III Trials
University of Oxford, UK (with AstraZeneca)
The investigators working on chimpanzee adeno (ChAd)- vectored vaccine platform developed ChAdOx1 nCoV-19, encoding the spike protein of SARS -CoV-2, which elicited robust humoral and cell-mediated response rhesus macaques [50]. The candidate vaccine was tested on more than 1000 human volunteers and based on the results [51], had entered into the Phase III trials. In the meantime, University of Oxford and its commercial arm Oxford Bio Medica [52,53] teamed up with Astra Zeneca and Vaccine Manufacturing and Innovation Centre to manufacture large quantities of the vaccine [54], which was named asAZD1222.
Sinovac Biotech, China
Sinovac Biotech Ltd., Beijing, China and their collaborators together had developed a pilot-scale production of a purified inactivated SARS-CoV-2 virus vaccine candidate (PiCoVacc) for protection against SARS-Co V-2. The vaccine formulation had induced virus-specific neutralizing antibodies in mice, rats and non-human primates. The antibodies produced neutralized ten representative SARS-CoV-2 strains. These results suggested a possible broader neutralizing ability against multiple numbers of SARS-CoV-2 strains. Macaques after vaccination with two different doses (3 μg or 6 μg per dose), on challenge provided partial or complete protection [55]. The vaccine has entered into Phase III trials.
Wuhan Institute
The Wuhan Institute of Biological Products, China (a government institute) had developed an inactivated SARS-Co V-2 vaccine in its manufacturing establishment. In China, the government owned manufacturing companies dominate [56,57] its vaccine manufacturing establishments.
Beijing Institute
Another Chinese government institutes, the Beijing Institute of Biological Products, China had also developed an inactivated SARS-Co V-2 vaccine.
China has deployed its inactivated SARS-Co V-2 vaccines developed [58] by the Wuhan Institute of Biological Products and the Beijing Institute of Biological Products on the Chinese recipients in large numbers but has not yet published their results of safety and efficacy. Both the candidate vaccines of Wuhan Institute and Beijing Institute are in Phase III clinical trials.
Moderna Inc., USA
Moderna Inc., USA a pioneering science-based company making top class [59] scientific publications had developed an mRNA platform and is presently working onm RNA based SARS-Co V-2 vaccines on a battle speed. Their SARS-Co V-2 vaccine is designated as m RNA-1273. The company had produced its first batch of m RNA-1273 vaccine doses on 07 February 2020, and after receiving US FDA approval commenced human trial on16 March 2020 on 45 healthy volunteers. On the basis of the results of safety [60], the vaccine entered into Phase-III clinical trials. Moderna also teamed up with Lonza, Switzerland, ensuring stable long-term production [61] of the candidate vaccine.
Bio N Tech, Germany (with Pfizer and Fosun Pharma, China)
In an announcement on May 05, 2020 Pfizer and Biopharmaceutical New Technologies (BioNTech) had mentioned about [62] the first –dose participants who had been injected with the COVID-19 mRNA Vaccine in the BNT162 vaccine program in the U.S.A. The COVID-19 mRNA vaccine is a lipid nanoparticles-encapsulated m RNA stretch designed to translate the immunogenic SARS-Co V-2 proteins against which antibodies are to be raised. The immune response and the safety studies have been completed and the candidate has entered into Phase III trial.
Phase II Trials
Can Sino Biologics, China
The scientists of Can Sino Biologics, Tianjin, China and other eminent scientists from multiple institutes of China had worked together and reported the initial findings on the post-vaccination human-safety, tolerability, and immunogenicity of their recombinant adenovirus type-5 (Ad5) vectored COVID-19 vaccine at 28 days after applying the dose. One hundred eight healthy individuals participated in the trial. There was no serious adverse event. The vaccine expresses the spike glycoprotein ‘S’ of a SARS-CoV-2 strain. The initial findings of the human results were satisfactory [63]. The candidate vaccine has entered into Phase II.
Anhui ZhifeiLongcom
Anhui ZhifeiLongcom Biologic Pharmacy Co. Ltd, China. which is a wholly owned subsidiary of Chongqing Zhifei Biological Products Co. Ltd., China had developed a DNA-based novel SARS-CoV-2 protein subunit vaccine produced in CHO cell lines, and had submitted information about their conducting Phase II clinical trials [64]. The trial was a parallel assignment for Phase I and Phase II studies. The candidate vaccine was developed jointly by Anhui Zhifei and the Institute of Microbiology under the Chinese Academy of Sciences [65]. Presently, the candidate is in Phase II stage.
Phase I/II
Institute of Medical Biology
Institute of Medical Biology (IMB), Chinese Academy of Medical Sciences in collaboration with West China Second University Hospital Yunnan Center for Disease Control and Prevention had submitted information [66] about their inactivated SARS-CoV-2 vaccine for the conduct of Phase I and Phase I/II trials. In the meantime, IMB had conducted pilotscale production of an inactivated SARS-CoV-2 vaccine candidate (BBIBP-CorV) and had tested the efficacy of the vaccine in mice, rats, guinea pigs, rabbits, and nonhuman primates (cynomolgus monkeys and rhesus macaques) and found that the vaccine had its capacity to generate high levels of neutralizing antibodies titers. The vaccine had also provided highly efficient protection against SARS-CoV-2 intra challenge in rhesus macaques. Further, the vaccine did not manifest detectable antibody-dependent enhancement of infection. The candidate vaccine had acceptable genetic stability for manufacture [67].
Inovio Pharmaceuticals, USA
The entrepreneurs from Inovio Pharmaceuticals, USA ( Inovio) along with the Coalition for Epidemic Preparedness Innovations (CEPI), the Korean Institute of Health and Social Welfare (KIHSW) and International Vaccine Institute(IVI)came together to develop SARS Co V-2 vaccine. Inovio had their previous experience of developing a synthetic DNA vaccine targeting the MERS coronavirus spike protein; the team has now built a synthetic DNA-based vaccine candidate, targeting the spike protein (S) of SARS-CoV-2 virus. The engineered construct named as INO-4800 is delivered through intradermal route using an electroporation. The vaccine was tested [68] on mice and guinea pigseliciting satisfactory immunological responses. Further, the candidate vaccine was assessed for immunogenicity and anamnestic protective efficacy in rhesus macaques [69]. Vaccination with INO-4800 induced T cell responses and neutralizing antibody responses against the SARS-CoV-2 spike proteins. The work is being carried out together with various components being planned and carried out in Korea and USA simultaneously. It has entered into Phase I/II.
Takara Bio
Takara Bio Inc [70], Japan in partnership with Osaka University and AnGes Co. Japan, Ltdis involved in the manufacture and testing of their DNA plasmid vaccine with their adjuvant along with their jet injection delivery device, which is currently being evaluated in preclinical studies. The vaccine is delivered through the intramuscular route and is in Phase I/II trial [71].
Bharat Biotech
Bharat Biotech [72] had mentioned that it had developed an inactivated candidate vaccine by the name COVAXIN in collaboration with the Indian Council of Medical Research (ICMR) -National Institute of Virology (NIV) Pune. COVAXIN is based on the use of a virus clade isolated from an Indian patient by the National Institute of Virology, Pune. The vaccine was being manufactured in Bharat Biotech's BSL-3 (Bio-Safety Level 3) high containment facility. The vaccine received the approval of the Drugs Controller General of India ( DCGI) for Phase I and II Human Clinical Trials and the trials had commenced across India from July, 2020.The trial is in Phase I/II stage.
Zydus Cadila
Zydus Cadila had mentioned that in its Vaccine Technology Centre at Ahmedabad, the company has developed a recombinant DNA vaccine platform where using a nonreplicating and non-integrating plasmid carrying the gene of interest can be expressed. The biosafety requirements for handling the technology is only of BSL-1 type the plasmid DNA vaccine candidate namely ZyCo V-D has successfully completed the preclinical phase of research and has received [73] permission for initiating Phase I /II human clinical trials from the Indian DCGI-Central Drugs Standard Control Organization (CDSCO). The company planned to start human clinical trial from July 2020. Zydus Cadila generated the preclinical safety and efficacy data on small animal in mice, rabbits, guinea pigs and rats; these animals developed antibodies [74] against the virus andthe animals could tolerate the plasmid vaccine.
Genexine Inc
Genexine Inc, South Korea took up the project of developing its DNA vaccine expressing the SARS-CoV-2 S-protein antigen. The vaccine candidate [75] was being developed through a consortium comprising Genexine, Binex, International Vaccine Institute, Genbio, the Korea Advanced Institute of Science and Technology and Pohang University of Science and Technology. The efforts were supported by the South Korean government. The vaccine is I Phase I/II evaluation [76] stage.
Novavax Inc
Novavax Inc., Gaithersburg, Maryland, USA had produced a vaccine candidate which was engineered from the genetic sequence of coronavirus spike protein COVID-19 which was a piece of recombinant protein , combined with their nanoparticle technology to generate the injectable antigen composite. The antigen composite was further combined with their proprietary adjuvant (Matrix-M) to make the vaccine formulation. The vaccine was named as NVX-CoV2373. Efficacy data generated in animal studies showed that the vaccine was highly immunogenic.
In March 2020, Novavax entered into an agreement with Emergent Bio Solutions for obtaining their formulations manufactured under Good Manufacturing Practices for conducting further trials. Subsequently, Phase I / II had been initiated on 130 healthy adults. The human trial began [77] in May 2020.
Kentucky Bio Processing
Kentucky Bio Processing, Inc, USA a subsidiary biotech unit of British American Tobacco (BAT), UK had developed [78] a protein subunit vaccine, expressed in genetically engineered tobacco plants; the recombinant protein was being isolated from the leaves. The recombinant protein was designed based on the protein sequence information of the receptor-binding domain of the S-1 spike protein of SARS-CoV-2 virus. The vaccine is presently in Phase I/II, first-in-human trial stage [79].
Arcturus Therapeutics
Arcturus Therapeutics, Inc., USA is a company engaged in the development of clinical-stage mRNA medicines and vaccines. They have developed their candidate LUNAR –COV19 vaccine which is a RNA vaccine formulation containing their designed self-amplifying mRNA with a proprietary nanoparticle delivery system named LUNAR. Arcturus Therapeutics has teamed up with Duke-NUS Medical School, Singapore to develop the LUNAR –COV19 vaccine further for human use [80]. They have received approval to precede with Phase I/II Clinical Trial and the work is in progress [81].
Johnson and Johnson
Johnson and Johnson had announced on 11th February 2020 that it had entered into a partnership with the Biomedical Advanced Research and Development Authority (BARDA) of the US Department for Health and Human Service (HSS) to commit more than US$1bn to co-fund a research project to develop an effective COVID-19 vaccine [82]. On 30th March 2020, J and J [83] announced that it had been working with BARDA. JandJ ’ s vaccine is human adenovirus vector based (adenovirus Ad26).The product is developed on its RandD platform, known as the AdVac platform, in collaboration with BARDA. In the US Government’ s Operation Warp Speed initiative [84] it was estimated that J and J’s COVID-19 vaccine would enter into Phase I/II a trials from July 2020. The latest position is not known.
Phase I
Imperial College
Imperial College London (ICL) from the Department of Infectious Disease developed [85] a candidate which is a self-amplifying recombinant RNA piece and works by producing a recombinant protein found on the surface of coronavirus, once the vaccine is injected into the muscles of the recipients. The recombinant protein elicits immunological responses in both the Th-1 and Th-2 pathways and triggers a protective immune response. In order to facilitate large-scale production, ICL had formed a new enterprise by the name Vac Equity Global Health (VGH). This enterprise shall be responsible for bringing out the COVID-19 vaccine of ICL to the world. VGH shall be supported by ICL and Morningside Ventures, Hong kong (a venture capital company).In the meantime, the COVID-19 vaccine candidate had entered into Phase I human trials [86,87] in June 2020 with 300 volunteers, participating in the trial. In October, 2020 further efficacy trial involving 6,000 people is planned.
Gamaleya National Research
Gamaleya National Research Centre of Epidemiology and Microbiology (GNRCEM), Russia, had announced in May 2020 that it had developed [88,89] the vaccine against SARS-CoV-2. GNRCEM is a Russian state institute. The vaccine construct is a genetically engineered adenovirus vector construct containing a piece of genetic material inserted into the DNA of the adenovirus. The recipient of the vaccine gets an immunogenic protein of SARS-CoV-2, translated by the recombinant adenovirus and elicits immunological responses. After completing the animal trials, the candidate vaccine was used on human volunteers in June 2020. On July 10, 2020, the Russian Ministry of Defense announced that it started the final stage of the coronavirus vaccine clinical trials of the vaccine developed by GNRCEM. On July 12, 2020 it was announced [90] that the GNRCEM vaccine had successfully completed the safety studies. An open two stage non-randomized Phase 1 study on healthy volunteers [91] started in June 2020. The vaccine project was funded by the Russian Direct Investment Fund (RDIF).
Clover Biopharmaceuticals
Clover Biopharmaceuticals [92] (also known as Sichuan Clover Biopharmaceuticals), Chengdu, China is a research-based biotechnology company. Their Australian unit by the name Clover Biopharmaceuticals AUS Pty [93], had provided Information on a proposal for a Phase 1, randomized, doubleblind, placebo-controlled novel recombinant SARS-CoV-2 trimeric S protein subunit vaccine for COVID-19.The study started in June 2020. Clover had teamed up with GSK [94] and Dynavax [95] to have access to the proprietary adjuvants of these companies for use in Clover’s vaccine candidate.
Vaxine Pty
Vaxine Pty Ltd., Adelaide, Australia announced [96] in May 2020 that the company had teamed up withMedytox Inc., Korean to develop and commercialize their COVID-19 vaccine which will be marketed by the trade name COVAX-19. COVAX-19 formulation is based on use of the proprietary adjuvant Advax of Vaxine Pty Ltd, combined with a recombinant SARS-CoV-2 spike protein subunit. Animal studies using the candidate vaccine has already been initiated in Australia and USA. Presently trials are in Phase I.
University of Queensland
University of Queensland (UQ), Australia and Aussie biotech CSL Ltd. (CSL), Australia teamed up [97] with Coalition for Epidemic Preparedness Innovations (CEPI) to accelerate development, manufacture and distribution of a COVID-19 vaccine which was developed by the UQ. The candidate is a protein subunit vaccine. The candidate is in Phase I evaluation [98].
CureVac
CureVac, Tübingen, Germany is a biopharmaceutical company [99] which specializes in developing therapies based on mRNA. The company has special interest on developing vaccines for infectious diseases, rare diseases and to treat cancer. The company obtained financial support from the Coalition for Epidemic Preparedness Innovations (CEPI) to develop the candidate vaccine. It had subsequently obtained [100,101] approval from the German Health Authority Paul-Ehrlich- Institute (PEI) and the Belgian Federal Agency for Medicines and Health Products (FAMHP) for conducting Phase I trial of their vaccine candidate, named CVnCoV. The product is an mRNA vaccine, which encodes chosen portions of the spike protein of SARS-CoV-2 and is formulated as lipid nanoparticles. The Phase I study began in June 2020.
Yunnan Watson
Yunnan Watson Biotechnology Co., Ltd. of Yunnan Walvax Biotechnology, Yunnan, China along with Academy of Military Medicine, Academy of Military Science of the Chinese People's Liberation Army, Beijing ,China had obtained approval [102,103] for the Phase I clinical trial to evaluate the safety, tolerability and preliminary immunogenicity of different doses of their novel coronavirus mRNA vaccine known by the name ARCoV,
Medicago Inc,
Medicago Inc, Canada is a biopharmaceutical company which had produced a Virus-Like Particle (VLP) of the coronavirus using proprietary genetically modified plant-based technology [104]. The company had earlier demonstrated how, using their plant-based platform candidate vaccines for both H1N1 influenza and Ebola could be developed. The Phase I trial [105] of the intramuscular injectable candidate vaccine was started in July 2020.
Medigen Vaccine
Medigen Vaccine Biologics Corporation (MVBC), a subsidiary of Medigen Biotechnology Corporation, Taiwan specializes in cell-cultivation platform technology and had developed a novel cell-based recombinant protein subunit vaccine [106] against SARS-CoV-2. MBVC had teamed up with Dynavax to use the latter’s proprietary adjuvant. The vaccine is going to Phase I trial from September 2020.
Concluding Remarks
In the existing situation of global pandemic, the vaccination of people is a crying public health priority. Vaccines can reduce the morbidity and mortality associated with COVID-19 disease. Vaccines should not only be safe and effective but should also be abundantly available at affordable prices, especially in poorer settings of the world. Innovative philanthropic initiatives are called for from international organizations to work with local governments to ease access.
Since the development of an effective vaccine against COVID-19 disease is moving at an unprecedented speed and since a large number of people are yet at risk of contracting the disease, there would be a large number of people who would be planning to receive a COVID 19 vaccine at the earliest. Presently, although a sizable number of vaccine candidates are being evaluated, none is yet approved. But individuals may like to use an experimental vaccine on a “compassionate use” program risking their safety. There has therefore to be adequate safeguards from governments, regulators, political system, the medical doctors and the clinicians, the health care organizations and others to adequately bring out information to the public about the status of a particular product for pre-licensure use by the vaccine recipients.
Acknowledgements
The authors acknowledge Ms. Deepali Ghosh, the other partner of Sompradip Publishers and Consultants, for encouraging and providing support to complete the manuscript.
Conflict of Interest
The authors have no conflict of interest regarding the content of this article.
REFERENCES
- Ghosh PK. Tuberculosis-prone countries and resistance to COVID-19. MGM J Med Sci.2020; 7(1): 31.
- SARS (Severe Acute Respiratory Syndrome) – WHO. 2020.
- Porcheddu R, Serra C, Kelvin D, Kelvin N, Rubino S. Similarity in case fatality rates (CFR) of COVID-19/SARS-COV-2 in Italy and China. J Infect.2020; 14(02): 125-128.
- Human Coronavirus Types | CDC. 2020.
- Wikipedia contributors. Severe acute respiratory syndrome coronavirus. In Wikipedia, The Free Encyclopedia. 2020.
- Auwaerter P. Coronavirus.2020
- Zhou P, Yang X, Wang XG, Hu B, Zhang W. Si H, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579: 270-273.
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. Severe acute respiratory syndrome coronavirus 2 isolate Wuhan-Hu-1, complete genome. Nature. 2020; 579(7798): 265-269.
- Fan W, Su Z, Bin Y, Yan-Mei C, Wen W, Zhi-Gang S, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020; 579(7798): 265-269.
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003; 426(6965): 450-454.
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nature medicine. 2005; 11(8): 875-879.
- Bertram S, Glowacka I, Müller MA, Lavender H, Gnirss K, Nehlmeier I, et al. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J virol. 2011; 85(24): 13363-13372.
- Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020; 5(4): 562-569.
- Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences. 2020; 117(21): 11727-11734.
- Enjuanes Sánchez L, Zúñiga Lucas S, Castaño-Rodríguez C, Gutierrez-Alvarez J, Cantón J, SoláGurpegui I. Chapter eight-Molecular basis of Coronavirus virulence and vaccine development.
- Tseng CT, Sbrana E, Iwata-Yoshikawa N, Newman PC, Garron T, Atmar RL, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PloS one. 2012; 7(4): e35421.
- Iwata-Yoshikawa N, Uda A, Suzuki T, Tsunetsugu-Yokota Y, Sato Y, Morikawa S, et al. Effects of Toll-like receptor stimulation on eosinophilic infiltration in lungs of BALB/c mice immunized with UV-inactivated severe acute respiratory syndrome-related coronavirus vaccine. J virol. 2014; 88(15): 8597-8614.
- Chen WH, Strych U, Hotez PJ, Bottazzi ME. The SARS-CoV-2 vaccine pipeline: an overview. Current tropical medicine reports. 2020; 3: 1-4.
- Tirado SM, Yoon KJ. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003; 16(1): 69-86.
- Wikipedia contributors. Antibody-dependent enhancement. In Wikipedia, The Free Encyclopedia. 2020.
- Hanney SR, Wooding S, Sussex J, Grant J. From COVID-19 research to vaccine application: why might it take 17 months not 17 years and what are the wider lessons?. Health Res Policy Syst. 2020; 18(1): 1-0.
- Wang Q, Wong G, Lu G, Yan J, Gao GF. MERS-CoV spike protein: targets for vaccines and therapeutics. Antiviral Res. 2016; 133: 165-177.
- Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. BioRxiv. 2020.
- Sun. Cell Host and Microbe, Elsevier Inc.2020; 28: 124–133.
- O’Callaghan KP, Blatz AM, Offit PA. Developing a SARS-CoV-2 vaccine at warp speed. JAMA. 2020.
- Hargreaves B., June 09, 2020, Oxford Biomedica adds two suites to manufacture AZ vaccines, 2020.
- Your questions answered: How Merck is responding to the .2020.
- IAVI and Merck Collaborate to Develop Vaccine Against SARS-CoV2, 2020.
- World Health Organization. DRAFT landscape of COVID-19 candidate vaccines. World. 2020.
- Akst J. COVID-19 vaccine frontrunners. The Scientist. 2020.
- Le TT, Andreadakis Z, Kumar A, Roman RG, Tollefsen S, Saville M, Mayhew S. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020; 19(5): 305-306.
- Funk CD, Laferrière C, Ardakani A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front Pharmacol. 2020; 11: 937.
- Bartsch SM, O'Shea KJ, Ferguson MC, Bottazzi ME, Wedlock PT, Strych U, et al. Vaccine Efficacy Needed for a COVID-19 Coronavirus Vaccine to Prevent or Stop an Epidemic as the Sole Intervention. Am J Prev Med. 2020.
- Tulane receives $1 million to assist in fighting COVID-19, 2020.
- INOVIO Initiates Phase 1 Clinical Trial Of Its COVID-19 ..., New2020.
- Kim E, Erdos G, Huang S, Kenniston TW, Balmert SC, Carey CD, Raj VS, et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020; 102743.
- Altimmune Launches Clinical Trial of T-COVIDTM, an. 2020.
- Intravacc Partners With WageningenBioveterinary Research. 2020.
- Evaluating the Safety, Tolerability and Immunogenicity. 2020.
- Vaxart Announces Selection of its Oral COVID-19 Vaccine Lead Candidate, 2020.
- Tableted COVID-19 Therapeutic Vaccine (COVID-19), 2020.
- Immunity and Safety of Covid-19 Synthetic Minigene Vaccine. 2020.
- SARS-CoV-2 Vaccine – AIVITABiomedical, 2020.
- Phase Ib-II Trial of Dendritic Cell Vaccine to Prevent COVID-19 in Adults, 2020.
- Valo Therapeutics to Support Development of a Pan – Coronavirus Vaccine, -News and Press Releases, 2020.
- Cauchon NS, Oghamian S, Hassanpour S, Abernathy M. Innovation in Chemistry, Manufacturing, and Controls—A Regulatory Perspective From Industry. Daru. 2019; 108(7): 2207-2237.
- Valo Therapeutics acquires PeptiENV technology from the University of Helsinki. 2020.
- Dai S, Wang H, Deng F. Advances and challenges in enveloped virus-like particle (VLP)-based vaccines. J Immunol Sci. 2018; 2(2): 36-41.
- Kirchmeier M, Fluckiger AC, Soare C, Bozic J, Ontsouka B, Ahmed T, et al. Enveloped virus-like particle expression of human cytomegalovirus glycoprotein B antigen induces antibodies with potent and broad neutralizing activity. Clin Vaccine Immunol. 2014; 21(2): 174-80.
- van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. 2020.
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet. 2020.
- Wikipedia contributors. Oxford BioMedica. In Wikipedia, The Free Encyclopedia, 2020.
- Blankenship K. AstraZeneca's COVID-19 vaccine enters phase 2/3 clinical trial, 2020.
- Hargreaves B., June 09, 2020, Oxford Biomedica adds two suites to manufacture AZ vaccines, 2020.
- Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020.
- Ghosh PK. Human vaccines industry in China, 2019: Part—I. MGM J Med Sci. 2020; 7(1): 35.
- Ghosh PK. Human vaccines industry in china, 2019: Part II. MGM J Med Sci. 2020; 7(2): 86.
- China Coronavirus Vaccine: Vaccine could be ready by 2020.
- Moderna, Inc. | Home. 2020.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020.
- Bell J, 8 June 2020, Moderna timeline: How the biotech firm has moved alongside pharma giants in Covid-19 vaccine race. 2020.
- Hub IA. Category: Intellectual Property. 2020.
- Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. The Lancet. 2020.
- Clinical Study of Recombinant Novel Coronavirus Vaccine,2020.
- China greenlights new COVID-19 vaccine candidate for 2020.
- Safety and Immunogenicity Study of an Inactivated SARS-CoV-2 Vaccine for Preventing Against COVID-19, 2020.
- Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, et al Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020.
- Smith TR, Patel A, Ramos S, Elwood D, Zhu X, Yan J, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nature communications. 2020; 11(1):1-3.
- Patel A, Walters J, Reuschel EL, Schultheis K, Parzych E, Gary EN, Maricic I, et al. Intradermal-delivered DNA vaccine provides anamnestic protection in a rhesus macaque SARS-CoV-2 challenge model. bioRxiv. 2020.
- COVID-19 vaccine development: the quest to dethrone the evil 2020.
- Study of COVID-19 DNA Vaccine (AG0301-COVID19) 2020.
- Bharat Biotech, 2020.
- Kaur SP, Gupta V. COVID-19 Vaccine: A comprehensive status report. Virus Research. 2020; 198114.
- Coronavirus Vaccine: India's second COVID-19 vaccine produced by Zydus Cadila cleared for human trials,
- TIMESOFINDIA.COM, created: 2020; 10:55.
- South Korea's Genexine begins phase I/IIa trials for COVID-19, 2020.
- Safety and Immunogenicity Study of GX-19, a COVID-19, 2020.
- Novavax – Creating Tomorrow's Vaccines Today. Novavax.com, 2020.
- BAT working on potential COVID 19, 2020. -British American Tobacco, 2020.
- KBP-201 COVID-19 Vaccine Trial in Healthy Volunteers. Arcturus Therapeutics and Duke-NUS Received Approval to, 2020.
- Phase 1/2 Ascending Dose Study of Investigational SARS-CoV-2 Vaccine ARCT-021 in Healthy Adult Subjects. 2020.
- Johnson and Johnson Announces Collaboration with U.S. 2020.
- Johnson and Johnson Announces a Lead Vaccine Candidate for, 2020.
- CanSino’s, JandJ’s Covid-19 vaccines may be stifled by pre-existing antibodies while AstraZeneca’s, ReiThera’s may need booster. GlobalData Healthcare; 2020.
- Angus T. In pictures: the Imperial lab developing a COVID-19 vaccine, 2020.
- ISRCTN17072692: Clinical trial to assess the safety ISRCTN. 2020.Scheuber A., Imperial social enterprise to accelerate low-cost COVID-19 vaccine, 2020.
- Russia’s COVID-19 Vaccine Successfully Tested. 2020.
- Russian COVID-19 Vaccine, Fact checked by Robert Carlson R, 2020.
- Russia successfully completes human trials on India Today. 2020.
- An Open Study of the Safety, Tolerability and Immunogenicity of the Drug "Gam-COVID-Vac" Vaccine Against COVID-19, 2020.
- Clover Biopharmaceuticals. Clover Biopharmaceuticals AUS Pty Ltd, 2020.
- SCB-2019 as COVID-19 Vaccine -Full Text View. 2020.
- Clover and GSK announce research collaboration to evaluate. 2020.
- Dynavax and Clover Biopharmaceuticals Announce Research. 2020.
- Vaxine announces COVID-19 vaccine collaboration with Medytox News, Biospectrum Asia Edition. 2020.
- CSL, University of Queensland partner with CEPI to bring, 2020.
- ANZCTR Trial Review, English -CureVac, 2020.
- CureVac to trial Covid-19 vaccine in Germany and Belgium,
- NCT04449276 -Clinical Trials, China's first COVID-19 mRNA vaccine approved for clinical. 2020.
- Chinese Clinical Trial Register (ChiCTR) 2020.
- COVID-19 Programs –Medicago. 2020.
- Safety, Tolerability and Immunogenicinity of a Coronavirus. 2020.
- Vaccine Medigen Biotech Corp. 2020.
- Dynavax and Medigen Announce Collaboration to Develop. 2020.
- A Phase I, Prospective, Open-Labeled Study to Evaluate the. 2020.
- Lurie N, Sharfstein JM, Goodman JL. The development of COVID-19 vaccines: safeguards needed. JAMA. 2020.
Citation: Ghosh S, Ghosh PK (2020) Effective SARS-CoV-2 Vaccines on the Horizon. J Vaccines Vaccin. 11:428. DOI: 10.35248/2157-7560.20.11.428
Copyright: © 2020 Ghosh S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

