Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 16, Issue 4
Dual Role of Salicylic Acid in Inhibiting Xanthomonas oryzae pv. Oryzae and Promoting the Growth of Rice Plants
Ahmed SA El-Shakh1*, Mohammed EA Jado1, Hatim BE Elfadni1, Mamoun A Homaida2, Yassin Haranb2 and Alsadig Yahya22Department of Food Science and Technology, Faculty of Agriculture and Natural Resources, University of Bakht Al-Ruda, Ed Dueim, Sudan
Received: 13-Aug-2024, Manuscript No. JBP-24-26747; Editor assigned: 15-Aug-2024, Pre QC No. JBP-24-26747 (PQ); Reviewed: 29-Aug-2024, QC No. JBP-24-26747; Revised: 14-Jun-2025, Manuscript No. JBP-24-26747 (R); Published: 21-Jun-2025, DOI: 10.35248/2155-9597.25.16.555
Abstract
Salicylic Acid (SA) plays an important role as an endogenous signal in mediating plant defense responses against pathogens. This study investigated the effects of SA on biofilm formation by Xanthomonas oryzae pv. oryzae (Xoo) and its ability to induce systemic resistance in rice against Bacterial Leaf Blight (BLB). Results demonstrated that treating rice plants with 0.5 mM SA significantly suppressed the growth and biofilm formation of Xoo. Transmission Electron Microscopy (TEM) confirmed that SA treatment caused noticeable changes in bacterial cell morphology, including swelling and wall alterations. In greenhouse experiments, SA inhibited the pathogen by 50.45% and increased plant height, fresh weight, and dry weight in treated rice plants. Additionally, SA application enhanced the activity of defense-related enzymes such as Polyphenol Oxidase (PPO), Peroxidase (POD), Phenyl Ammonia Lyase (PAL), Superoxide Dismutase (SOD), and Catalase (CAT), indicating that SA improves systemic resistance in rice plants against BLB. These findings highlight the dual role of SA in inhibiting pathogen growth and promoting plant growth by activating defense mechanisms. This study provides valuable insights into the application of SA as a potential strategy for managing bacterial leaf blight and improving rice plant health under biotic stress conditions.
Keywords
Salicylic acid; Xanthomonas oryzae; Bacterial leaf blight; Rice defense mechanisms; Biofilm inhibition
Introduction
Rice is the main dietary energy source among cereals and the staple food for over 2.7 billion people in Asia, where 90% of the world's rice is cultivated and consumed. Pests and diseases are the primary constraints in rice production. Bacterial Leaf Blight (BLB) caused by Xanthomonas oryzae pv. oryzae is a severe disease affecting rice production globally, leading to substantial economic losses. Xoo, like other Xanthomonas species, produces various virulent factors, including Extracellular Polysaccharides (EPS), lipopolysaccharides, adhesins, cell wall degrading enzymes, type III effectors, and biofilm formation.
Biofilm is a self-produced matrix of extracellular polymeric substance, also known as slime, consisting of extracellular biopolymers in different structural forms. Quorum Sensing (QS) plays a vital role in reducing pathogenicity by controlling biofilm formation in the pathogen. In plants, signaling pathways are typically regulated by small-molecule hormones, including salicylic acid. When plants are attacked by pathogens, they synthesize a complex blend of hormones known as the hormone signature. This hormone signature is crucial in coordinating plant immune responses and determining the specific defense mechanism triggered. Interestingly, recent developments suggest that plant hormones may not only influence plant immune responses but also impact the pathogen's virulence machinery.
Salicylic Acid (SA) is a phenolic compound synthesized throughout plants via the phenylpropanoid pathway, which regulates resistance to bacterial, fungal, and viral pathogens. For example, a previous study reported that SA reduces the virulence of Agrobacterium tumefaciens by inhibiting the virA/G two-component system. Moreover, SA has been shown to decrease the production of various virulence factors, including motility, biofilm formation, and the production of extracellular signal molecules known as Autoinducers (AIs), in the human pathogen Pseudomonas aeruginosa. Several authors have also reported the antibiofilm properties of salicylic acid by downregulating total protease and elastase activities [1].
Salicylic acid is a crucial secondary signal in plants that activates defense genes when plants are attacked by pathogens. Multiple pathways regulate this signaling process. SA has been found to play important roles in various physiological processes and helps plants tolerate biotic and abiotic stresses. Previous studies have shown that SA can induce systemic acquired resistance and enhance disease resistance against viruses and fungi in pears, Arabidopsis, and cotton. However, there is limited information on how SA contributes to disease resistance in rice. Therefore, this study aims to investigate the role of SA in rice plants' defense against sss pv. oryzae (Xoo). The objectives are to examine the inhibitory effects of SA on Xoo growth and biofilm formation, observe changes in Xoo cell morphology using transmission electron microscopy, analyze the impact of SA on rice plant growth and defense-related enzyme activities in a greenhouse setting, and understand how SA enhances systemic resistance in rice against Bacterial Leaf Blight (BLB). We hypothesize that treatment with SA will significantly inhibit Xoo biofilm formation, alter bacterial cell morphology, promote rice plant growth, and activate defense-related enzymes, ultimately leading to increased resistance against BLB.
Materials and Methods
Salicylic acid preparation
Salicylic Acid (SA) used in this study was purchased from Sangon Company and dissolved in sterile deionized water. It was then serially diluted to obtain a solution with a final concentration of 0.5 mM. All the remaining chemicals and solvents used in the study were of analytical grade obtained from Sigma-Aldrich, USA [2].
Antibacterial activity of salicylic acid
The inhibitory activity of SA (0.5 mM) against Xoo was evaluated by measuring optical density (OD600) using the method of Wang et al. with some modifications. Briefly, 90 μL of LB was added to each well of a commercially available presterilized, polystyrene, flat-bottom 96-well plate. Then, 10 μL of pathogen cultural suspension (108 CFU/mL) was added to each well. After that, 100 μL of salicylic acid (0.5 mM) was added to each well. The plate was incubated at 28°C for 24 h without shaking. The negative control was detected by wells filled with pathogens (Xoo+LB). The OD600 of each well was measured after 24 h of incubation using a Micro Plate Photometer (Thermo Fisher Scientific Inc., Waltham, USA).
Inhibition of biofilm formation in Xoo
The capability of SA to inhibit the biofilm formation of Xoo was examined using 96-well microtiter culture plates, following the method of Nijland et al. In brief, Xoo was grown in LB medium for 12 h (108 CFU/mL) at 28°C. After that, 5 μL of the pathogen culture was added to 95 μL of pure LB in the 96-well plates, and the plate was incubated at 30°C for 24 h. Afterward, 100 μL of salicylic acid (0.5 mM) was added to the wells. Only LB medium was used in six wells selected for the negative control. Additionally, the plate was further incubated for 1 h at 30°C. All non-attached cells were removed by discarding the culture medium. The plate was rinsed four times in doubledistilled (ddH2O) gently and dried for 1 h. The attached biofilm material was stained with 1% Crystal Violet solution (CV) and incubated at room temperature for 25-30 min. The stained solution was then solubilized by adding 150 mL of 33% acetic acid, and its absorbance was measured at 570 nm [3].
TEM assay
In this experiment, 500 μL of Xoo (108 CFU/mL) was added to a sterile 10 mL tube containing 3 mL of LB and 3 mL of salicylic acid solution (0.5 mM). A tube containing 500 μL of Xoo with 6 mL LB served as the negative control. The samples were incubated on a rotary shaker (160 rpm) at 30 °C for 4 h. Each sample was then prepared for TEM following the procedure described by Helander et al. Briefly, the suspensions were centrifuged, washed, and transferred to new Eppendorf tubes containing 2.5% glutaraldehyde. The suspension was incubated overnight at 4°C. The samples were dehydrated using a graded series of ethanol (50, 70, 80, 90, 95, and 100%). Finally, the specimen was embedded in Spurr's medium at 70°C for 9 h. After polymerization, ultra-thin sections were cut with an LKB 8800 Ultra tome, stained with 2% (w/v) lead citrate and 2%(w/v) uranyl acetate, and analyzed using an electron microscope at an operating voltage of 75 kV [4,5].
Effect of salicylic acid on rice resistance to Xoo
To examine the biological control of BLB of rice, the greenhouse experiment tested the effectiveness of salicylic acid. Rice seeds were sterilized in a 2% sodium hypochlorite solution for 2 min and then washed five times with sterilized distilled water. The seeds were then soaked in salicylic acid (0.5 mM) for 8 h, while the control seeds were immersed in sterilized saline. After this treatment, the seeds were germinated in Petri dishes and incubated in a growth chamber at a temperature of 28°C for 3 days. Once germinated, the seeds were transferred to pots containing sterilized peaty soil and maintained under controlled greenhouse conditions (28°C during the day, 25°C during the night, and a relative humidity between 70 to 90%). After that, at the maximum tillering stage (20 days), the rice leaves were sprayed with a solution of SA (0.5 mM) and a saline solution. Two days later, three leaves from each of the six previously treated plants were inoculated with a suspension of Xoo strain PXO99, at a concentration of 108 Colony-Forming Units (CFU) per milliliter, using the leaf-clipping method. After 15 days, the length of the lesions and the inhibition rate of the inoculated leaves were measured and calculated. Two control treatments were considered: Control one (C1), which was treated only with the pathogen, and control two (C2), which was treated only with the sterile saline solution. After an additional 20 days from the second application, the effect of SA on plant growth, including plant height, fresh weight, and dry weight, as well as the Growth Promotion Efficacy (GPE%), was measured. The pots were arranged in a completely randomized block design with three replicates for each treatment and four plants per replicate [6].
Quantification of defense-related Enzymes
Total enzyme activities, Including Polyphenol Oxidase (PPO), Peroxidase (POD), Phenyl Ammonia Lyase (PAL), Total Superoxide Dismutase (SOD), and Catalase (CAT), were determined using a Bio-Rad SmartSpec TM 3000 spectrophotometer. Rice plant leaves were sampled for enzymatic analysis after 30 days. Three leaf samples (0.5 g) from each treatment were homogenized with a mortar and pestle in 5 mL of ice-cold 50 mM phosphate buffer solution at pH 7.8. The homogenate was then centrifuged at 12000 rpm for 20 min, and the supernatant fraction was preserved in small tubes. All operations were performed at 4°C.
Estimation of PPO activity
PPO activity was determined using the method of. 100 μL of extracted enzyme was incubated with 0.5 mL of 0.5 M catechol at 24°C for 2 min, followed by the addition of 2 mL of 0.05 M phosphate buffer (pH 7). The absorbance at 398 nm was measured using an ultraviolet spectrophotometer. The PPO absorbance was measured at 398 nm using the PPO unit of enzyme (PPO=0.01ΔOD398).
Estimation of POD activity
POD activity was assessed using guaiacol as a substrate. The reaction mixture contained 2 mL of guaiacol (8 mM) in 100 mM sodium phosphate buffer (pH 6.4), and 0.1 mL of crude extract. The mixture was incubated at 30°C for 30 min. After adding 1 mL of H2O2 (24 mM), the increase in absorbance at 460 nm was measured. The absorbance of POD was measured at 460 nm using the U460 unit of Enzyme (U460=0.01ΔOD460).
Estimation of PAL activity
PAL activity was determined using the protocol published by Assis et al. Briefly, 1 mL of 0.02 M L-phenylalanine and 2 mL of the PAL extracting buffer were incubated with 300 μL of enzyme extract at 24°C for 2 min. The mixture was then measured at 290 nm using an ultraviolet spectrophotometer. PAL activity was calculated using the formula U290= 0.01ΔOD290, where U290 represents the enzyme units at a wavelength of 290 [7].
Estimation of SOD activity
SOD activity was determined following the method described by GE et al. The reaction mixture consisted of 130 mM methionine, 75 mM NBT, 20 mM riboflavin, and 100 mM EDTA. Test tubes containing the reaction mixture were placed under a fluorescent lamp (40 W) at a distance of 30 cm and at a temperature of 25°C. Riboflavin and NBT were dissolved separately in distilled water. The spectrophotometer was turned on and allowed to warm up for 30 min before use, and then the absorbance was measured at 560 nm. Non-irradiated samples were used as controls.
Estimation of CAT activity
The CAT activity was assessed using the method described by Aebi. The assay mixture consisted of 0.1 mL of leaf enzyme extract, 0.1 mL of H2O2, and 2.8 mL of phosphate buffer at pH 7.8, making a total volume of 3 mL. Controls were included to detect the absence of enzyme extract. The CAT level was determined by measuring the decrease in absorbance at 240 nm, which indicated the disappearance of H2O2.
Statistical analysis
The data were analyzed using SAS software (SAS Institute, Cary, NC) with Analysis of Variance (ANOVA). To determine significant differences among the main treatments, a General Linear Model (GLM) procedure was applied. Individual comparisons between mean values were conducted using The Least Significant Differences (LSD) test (P<0.05).
Results
Antibacterial activity of salicylic acid against Xoo
Salicylic Acid (SA) exhibited significant antibacterial activity against Xoo in vitro (Figure 1). The viable populations of the pathogen treated with SA were lower than their respective control, indicating higher growth inhibition of Xoo by SA (0.61 OD at OD600) compared to the control (1.156 OD) (Figure 1).
Figure 1: Effect of salicylic acid (SA) 0.5 mM on in vitro growth of X. oryzae pv. oryzae. Results are expressed as OD reductions relative to control. Xoo (pathogen alone) represent negative control. Values show the means of six replicates with standard error. Different letters indicate significant differences between treatments at (P<0.05) by using the LSD test.
Inhibitory effect on biofilm formation of Xoo
The ability of Xoo to form a biofilm was significantly reduced when treated with SA (Figure 2). The biofilm data showed that SA had the highest anti-biofilm activity (0.052 OD) in the absorbance at 570 nm, resulting in an 83.87% inhibition of biofilm formation by Xoo compared to the control (Figure 2).
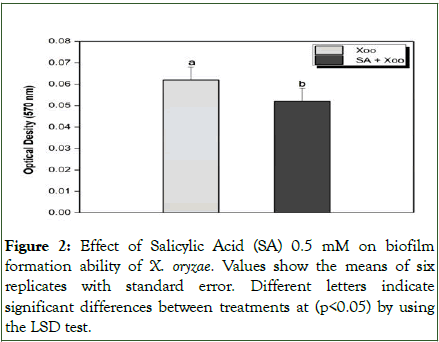
Figure 2: Effect of Salicylic Acid (SA) 0.5 mM on biofilm formation ability of X. oryzae. Values show the means of six replicates with standard error. Different letters indicate significant differences between treatments at (p<0.05) by using the LSD test.
Disruption of cell integrity in Xoo
In transmission electron micrographs, differences in cell morphology were visually observed between the SA-treated group and the control group (pathogen only) (Figure 3). The membranes of bacterial cells (Xoo) treated with 0.5 mM SA became swollen (Figure 3b), while untreated cells had intact and visible cell membranes (Figure 3a). Some cells of the treated Xoo bacteria exhibited wrinkled surfaces with numerous cracks, indicating a complete disturbance of the outer layer of cells. Furthermore, some SA-supplemented bacterial cells showed significant expansion and the formation of vacuole-like structures (Figure 3b).
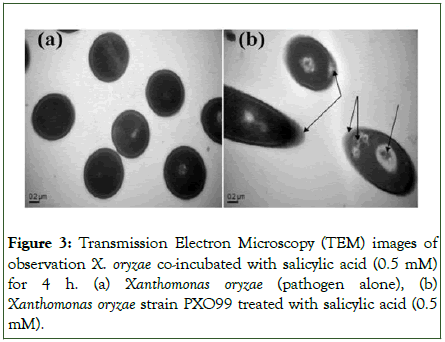
Figure 3: Transmission Electron Microscopy (TEM) images of observation X. oryzae co-incubated with salicylic acid (0.5 mM) for 4 h. (a) Xanthomonas oryzae (pathogen alone), (b) Xanthomonas oryzae strain PXO99 treated with salicylic acid (0.5 mM).
Effect of salicylic acid on Xoo and rice plant growth under greenhouse conditions
In this greenhouse study, the impact of SA on the lesion length of BLB and the inhibition rate of Xoo was evaluated. Rice plants treated with SA exhibited a significant reduction in the lesion length of BLB compared to untreated control plants. Additionally, the application of SA resulted in a significant increase in the inhibition rate of Xoo. The inhibition rate observed in rice seedlings treated with SA was 50.45% compared to the control (Table 1 and Figure 4). Moreover, plants treated with SA experienced a 14.61% increase in plant height (GPE). Furthermore, the fresh weights and dry weights increased by 17.82% and 25% (GPE), respectively, compared to the control (only Xoo) (Table 1 and Figure 4).

Figure 4: Image of rice plant growth and symptoms of bacterial leaf blight disease appeared on rice plant 15 days after inoculation. Xoo=plant treated only by Xanthomonas oryzae pv. oryzae (pathogenic control); C=plant treated only by saline solution (negative control); SA+Xoo=plant treated by salicylic acid and inoculated with pathogen.
Activities of enzymes
The study analyzed enzyme activities including Polyphenol Oxidase (PPO), Peroxidase (POD), Phenyl Ammonia Lyase (PAL), Total Superoxide Dismutase (SOD), and Catalase (CAT) using a spectrophotometer. The results showed significantly higher activity of defense-related enzymes against the Xoo pathogen. These enzymes were found to be remarkably higher in rice plant leaves treated with SA compared to the pathogen challenged and unchallenged controls (Figure 5a-e).
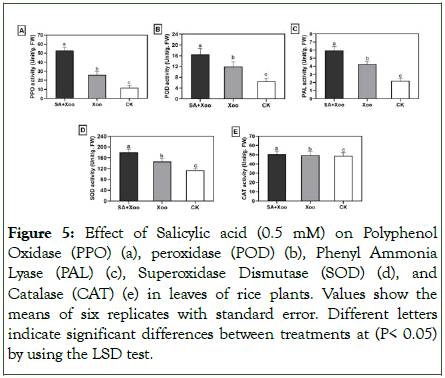
Figure 5: Effect of Salicylic acid (0.5 mM) on Polyphenol Oxidase (PPO) (a), peroxidase (POD) (b), Phenyl Ammonia Lyase (PAL) (c), Superoxidase Dismutase (SOD) (d), and Catalase (CAT) (e) in leaves of rice plants. Values show the means of six replicates with standard error. Different letters indicate significant differences between treatments at (P< 0.05) by using the LSD test.
Discussion
The interaction between salicylic acid and host plants has been intensively researched. SA can suppress plant pathogens and promote plant growth. Therefore, this study investigated the mechanisms of SA against bacterial leaf blight disease in rice caused by Xoo.
In the present study, SA (0.5 mM) significantly inhibited Xoo growth. The viable populations of the pathogen treated with SA decreased compared to the control (Figure 1). This is consistent with a previous study that reported the antimicrobial activity of SA against different human and plant pathogens. Similarly, SA showed substantial inhibition of bacterial growth in both liquid and solid medium against X. oryzae, further highlighting the antibacterial potential of SA.
SA exhibited remarkable antibiofilm activity against Xoo biofilm formation (Figure 2). Several researchers have reported SA's ability to inhibit the formation of pathogen biofilms. For example, SA inhibited the production of biofilm-associated enzymes by Pectobacterium carotovorum and Pseudomonas syringae pv. syringae. SA inhibits the production of virulence factors by Pseudomonas aeruginosa. Additionally, Xu et al. found that SA inhibited bacterial swimming while promoting EPS synthesis and upregulated various genes located both upstream (rpf gene cluster) and downstream (pilA) in the DSF signaling pathway, suggesting that signaling molecules may play a key role in determining the state of biofilm. Furthermore, SA affected swimming, twitching, and swarming motility in Pseudomonas aeruginosa, resulting in decreased biofilm formation.
Transmission electron microscope images revealed morphological changes in Xoo cells due to the application of SA (Figure 3). The wrinkled surfaces and swollen cell structures indicated that the cell layer of the Xoo cells was disordered, suggesting that SA altered cell morphology (Figure 3b). These changes may be caused by irreversible interaction of SA molecules with the cells. In contrast, no remarkable changes in cell structure were observed in untreated Xoo cells, indicating that no protein or DNA was released from the cytoplasm. This study provides clear evidence of SA's involvement in disrupting the outer membrane of Xoo. In the present study, the application of SA in the greenhouse reduced bacterial leaf blight in rice plants through a decrease in lesion length and wilting. Our findings strongly indicate that SA inhibited the growth rate of Xoo by 50.45% and increased plant height and weight compared to the Xoo control (Table 1 and Figure 4). These results are consistent with the findings of de Vleesschauwer et al., who reported that SA plays an important role in activating rice defenses against Xoo in the greenhouse. Another study showed that SA effectively controlled sheath blight disease caused by Rhizoctonia solani in rice plants in the greenhouse. These results suggest that the antimicrobial activity of SA is due to high levels of SA in the xylem, which restricts the growth and motility of Xoo and serves as an efficient defense mechanism for rice.
| Treatments | Lesion length (cm) | Inhibition rate (%) | Plant height (cm) | GPE% | Fresh weight (g) | GPE% | Dry weight (g) | GPE% |
| SA + Xoo | 9.83 ± 0.61 | 50.45 | 48.82 ± 0.61a | 24.54 | 7.24 ± 0.23a | 33.58 | 1.53 ± 0.02a | 45.71 |
| Control | - | - | 43.40 ± 0.82b | 10.71 | 6.12 ± 0.23b | 12.92 | 1.28 ± 0.10b | 21.9 |
| Xoo | 19.92 ± 0.66 | - | 39.20 ± 0.27c | - | 5.42 ± 0.19c | - | 1.05 ± 0.06c | - |
| Note: Data are presented as mean value ± standard error of three replicates, and each replicate contains four plants. Values with the different letters within each column indicate significant difference according to the LSD test at p<0.05. Xoo: Xanthomonas oryzae pv. oryzae. GPE: Growth Promotion Efficacy | ||||||||
Table 1: Effects of 0.5 mM salicylic acid on Xoo lesion length, inhibition rate, and rice plant growth parameters (fresh weight, dry weight, plant height, and GPE) in the greenhouse.
Furthermore, the enhanced growth characteristics observed in rice plants inoculated with Xoo and treated with 0.5 mM SA may be attributed to SA's effects on transcription and/or translation, leading to increased activity of various enzymes necessary for plant growth. SA also promotes cell division and cell enlargement. These findings are consistent with the work of other scientists who observed similar effects in rice plants inoculated with Rhizoctonia solani in the presence of SA [8].
Plant defense mechanisms are complex, involving multiple layers of defense against various pathogens. Plants utilize preformed physical and chemical barriers to hinder pathogen entry and infection. The results of our study demonstrate that treatments with SA significantly reduced bacterial leaf blight caused by Xoo in rice plants under greenhouse conditions. This effect may be attributed to the stimulatory properties of SA on Induced Systemic Resistance (ISR), as evidenced by increased activity of defense enzymes such as PPO, PAL, POD, SOD, and CAT. PPO is a copper-containing enzyme known to catalyze the oxidation of phenolics to more toxic quinones. PPO-generated quinones and Reactive Oxygen Species (ROS) can simultaneously perform a variety of defense-related functions. Additionally, PAL plays a crucial role in the biosynthesis of phenolics, phytoalexins, and lignin, which are key factors responsible for disease resistance. In the current work, it was revealed that SA treatment effectively enhanced the activities of PAL and PPO in rice leaves compared to the unchallenged control and pathogen control (Figure 5a and b). These results support previous reports that SA can inhibit various plant pathogens and diseases through the induction of ISR in plants. Previously observed that the application of SA increases PAL and PPO activities in melon.
These findings suggest that these changes may play a role in enhancing disease resistance in SA-treated leaves and protecting plants by triggering ISR [9].
The induction of ROS scavenging enzymes, such as POD, SOD, and CAT, is a common mechanism for detoxifying ROS synthesis during plant-pathogen interactions. In our results, the accumulation of POD, SOD, and CAT showed significantly higher levels in the SA treatment compared to the pathogenchallenged and unchallenged controls (Figure 5c-e). Previous studies have shown that SA-mediated ISR can enhance the induction of antioxidant enzymes like POD, SOD, and CAT, protecting against diverse pathogens. Similar results were observed in rice plants inoculated with Rhizoctonia solani under the influence of SA. The activities of these antioxidant enzymes reduced oxidative damage caused by pathogens, suggesting that SA may have a positive role in antioxidation through the upregulation of POD, SOD, and CAT activities. In conclusion, the results of this study provide evidence that enhancing the activity of these enzymes is essential for demonstrating a defense mechanism in plants against BLB after treatment with SA.
Conclusion
We can conclude that SA showed remarkable inhibition of the growth and biofilm formation of Xoo in vitro. Additionally, SA showed promise in controlling BLB disease and enhancing the growth of rice plants in the greenhouse. Moreover, SA induced systemic resistance in rice plants against BLB, as evidenced by increased activities of defense enzymes. Therefore, this data will support our successful approach to using SA in managing bacterial leaf blight and other phytopathogens.
Acknowledgments
The staff at the Laboratory of Crop Protection, Faculty of Agriculture and Natural Resources, University of Bakht Al-Ruda are highly acknowledged for their help and support.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126.
- Almoneafy AA, Kakar KU, Nawaz Z, Li B, Saand MA, Chun-lan Y, et al. Tomato plant growth promotion and antibacterial related-mechanisms of four rhizobacterial Bacillus strains against Ralstonia solanacearum. Symbiosis. 2014;63:59–70.
- Alyemeni MN, Hayat Q, Wijaya L, Hayat S. Effect of salicylic acid on the growth, photosynthetic efficiency and enzyme activities of leguminous plant under cadmium stress. Not Bot Horti Agrobot Cluj-Na. 2014;42(2):440–445.
- Assis JS, Maldonado R, Muñoz T, Escribano MI, Merodio C. Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biol Technol. 2001;23(1):33–39.
- Bandara MBK, Zhu H, Sankaridurg PR, Willcox MDP. Salicylic acid reduces the production of several potential virulence factors of Pseudomonas aeruginosa associated with microbial keratitis. Invest Ophthalmol Vis Sci. 2006;47(10):4453–4460.
[Crossref] [Google Scholar] [PubMed]
- Bari R, Jones JDG. Role of plant hormones in plant defence responses. Plant Mol Biol. 2009;69:473–488.
[Crossref] [Google Scholar] [PubMed]
- Beckers GJM, Spoel SH. Fine-tuning plant defence signalling: salicylate versus jasmonate. Plant Biol. 2006;8(1):1–10.
[Crossref] [Google Scholar] [PubMed]
- Bharwana SA, Ali S, Farooq MA, Ali B, Iqbal N, Abbas F, et al. Hydrogen sulfide ameliorates lead-induced morphological, photosynthetic, oxidative damages and biochemical changes in cotton. Environ Sci Pollut Res. 2014;21:717–731.
[Crossref] [Google Scholar] [PubMed]
- Büttner D, Bonas U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol Rev. 2010;34(2):107–133.
[Crossref] [Google Scholar] [PubMed]
Citation: El-Shakh SA, Jado MEA, Elfadni HBE, Homaida MA, Haranb Y, Yahya A (2025) Dual Role of Salicylic Acid in Inhibiting Xanthomonas oryzae pv. oryzae and Promoting the Growth of Rice Plants. J Bacteriol Parasitol. 16:555.
Copyright: © 2025 El-Shakh SA, et al. This is an open-access article distributed under the terms of the creative commons attribution license which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.

