Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 3
Detection of Intestinal Parasites in Stool Samples by Microscopy and Real-Time PCR in Children with Vulnerable Living Conditions in Dakar, Senegal
Souleye Lelo1*, Fatimata Ly2, Aminata Lam1, Cheikh Binetou Fall1, Issac Manga1, Fassiatou Tairou1, Khadim Sylla1, Magatte Ndiaye1, Doudou Sow1, Roger Tine1 and Babacar Faye12Department Service of Dermatology, Hospital Institute Hygiene and Social, Dakar, Senegal
Received: 02-Apr-2021 Published: 23-Apr-2021, DOI: 10.35248/2155-9597.21.12.398
Abstract
Background: Intestinal Parasitic Infections (IPIs) are considered a serious public health problem and widely distributed worldwide, mainly in urban and rural environments of tropical and subtropical countries. Globally, soil- transmitted helminths and protozoa are the most common intestinal parasites. Decreasing the prevalence of IPIs is one of the main aims of health services in these countries. This study was designed to determine the current status of IPIs in children with vulnerable living conditions by microscopy and PCR.
Methodology/main findings: A cross-sectional population-based survey was conducted. One stool sample per participant (n=253) was examined by direct smear, Formal-Ether Concentration (FEC), and real-time PCR. It was found that 17.39% harboured at least one helminth while 12.64% harboured two helminths or more. Among the microscopic techniques, FEC was able to detect the broadest spectrum of parasite species. However, FEC also missed a considerable number of infections, notably S. stercoralis and G. intestinalis. PCR outperformed microscopy in terms of sensitivity and range of parasite species detected.
Conclusion: It was shown that intestinal parasites, especially helminths were omnipresent in our population studies. Classical techniques such as FEC are useful for the detection of some intestinal helminth species, but they lack sensitivity for other parasite species. PCR can detect intestinal parasites more accurately but is generally not feasible in resource-poor settings, at least not in peripheral labs. Hence, there is a need for a more field-friendly, sensitive approach for on-the-spot diagnosis of parasitic infections.
Keywords
Helminths; Protozoa; RT-PCR; Vulnerable children
Introduction
Intestinal Parasitic Infections (IPIs) remain a public health problem in many communities, especially among children living in developing countries. It is estimated that more than 2 billion people worldwide are infected with IPI and more than half of the world's population is at risk of infection [1,2]. In sub-Saharan countries, 866 million people are infected with Soil-Transmitted Helminths (STH), the majority of these infections occurring in schoolchildren. The World Health Organization (WHO) estimates that 450 million people are sick [3,4]. In addition to morbidity and mortality, infections with intestinal parasites have been associated with growth retardation, underweight, physical weakness and poor school performance in schoolchildren [5,6]. As part of a long-term goal to eliminate intestinal parasitic infections as a public health problem by 2020, WHO has recommended Mass Drug Administration (MDA) with a single oral dose of mebendazole or albendazole given periodically to children preschool and school age [7,8]. This strategy is currently being implemented in many countries including Senegal. Microscopic examination of stool samples is the most widely used diagnostic approach for the detection of intestinal parasites. First of all, a direct microscopic examination is carried out by mixing a small amount of faeces with physiological sodium chloride solution (0.9%). Next, various stool concentration techniques based on the use of sedimentation or flotation with formalin-ether concentration technique are performed to increase sensitivity [9,10]. In addition, an accurate microscopic diagnosis depends on the experience of the laboratory microscopist and the concentration of parasitic material in the sample. Finally, certain species of parasites such as Entamoeba histolytica, Cryptosporidium sp and Strongyloides stercoralis, which are responsible for serious infections, are often misdiagnosed even when concentration techniques are used [11,12]. Although microscopic examination of stool specimens remains the gold standard for the diagnosis of parasitic infections, it is lengthy, laborious and requires substantial technical expertise. In contrast, rapid diagnostic methods such as Polymerase Chain Reaction (PCR)- based assays were developed to improve the sensitivity and specificity of detection of enteric parasites, including helminths, protozoa and microsporidia [13,14]. To overcome these shortcomings, molecular techniques were suggested as a complementary process and may be an alternative to microscopic examination. Indeed, the conventional real-time Polymerase Chain Reaction (PCR) has turned out to be sensitive and precise for the detection of helminths and intestinal protozoa [14]. These techniques have the advantage of detecting low levels of parasites, improving the identification of infected persons and evaluating treatment effects by quantification [15]. This Work is the first prospective, Senegal epidemiological study aiming at estimating the occurrence of three helminth Ascaris, Strongyloides and Trichocephale and one protozoan Giardia intestinalis. Microscopy and qPCR results were also compared.
Materials and Methods
This study was carried out in Koranic schools in the Dakar region between January and August 2018.
Study sites
A mapping of this area was prepared and the Koranic schools were randomly selected. In order to obtain a random and evenly geographically distributed, Koranic schools and a logistically and technically feasible laboratory sample size were selected. In this way, all participants, i.e. a total of 15 Koranic schools were approached to participate. In the field, these schools were identified thanks to the health district relays. Schoolchildren who did not provide enough faeces for all procedures were excluded from the study. Collection was done on 253 of the schoolchildren who gave informed consent and who provided enough faeces to be included in the study.
Microscopy
Stool samples were collected from all “Darras” (Koranic schools) members between 8 a.m. and 2 p.m. Arrived at the laboratory of the Faculty of Medicine the stools were immediately examined. Two well-trained microscopists followed the laboratory procedures and on average no more than 15 stool samples were processed per day with a double reading of the slide to ensure high quality microscopic results. Several approaches were used for the detection of cysts and oocysts of protozoa and of helminth eggs and larvae. Microscopic techniques included direct smear and the modified Ritchie Method. For the direct smear, ~2 mg of faeces was mixed with normal saline on a microscopy slide and examined for helminth eggs. Another ~2 mg of faeces was mixed with a drop of iodine and examined for protozoan cysts. For the modified Ritchie Method, one gram of fecal matter was thoroughly mixed with 8 ml of 10% formalin. An FPC filter with a 15 ml tube was attached to the tube containing this mixture. After filtering the suspension in the empty tube, 3 ml of ether was added to the filtrate. This mixture was then shaken vigorously for 1 minute and centrifuged at 500 × g for 2 minutes. A thick, unstained sediment frame was used for the detection of helminth eggs and larvae. For protozoan cysts, a thin moist layer of iodine-stained sediment was used.
Real time PCR assay
At the laboratory of the department of Parasitology (Faculty of Medicine, Cheikh Anta Diop University), an aliquot (~1 g) of each stool sample was sieved and mixed with 3 volumes of 96% ethanol for storage for performing Real-time PCR. Then the samples were stored at -20°C until the detection and quantification of parasitic DNA loads by real-time PCR. Isolation, amplification and detection of DNA were performed blind from previous microscopic results. DNA was extracted from the stool samples using a modified method of the Omega Bio-tek Extraction Kit [16,17]. For DNA isolation, 200 mg of faeces was collected on Eppendorf tubes of 1.5 ml and 30 μl of proteinase K and 250 μl of BL Buffer are added, then vortex for 15 seconds and incubate at 65°C for 10 minutes the pellet was washed with a solution with 260 μl of absolute ethanol, and vortex at maximum speed. The entire sample was transferred to the column, 500 μl of HBC Buffer were added followed by successive washings with 700 μl DNA washing buffer then a 100 μl Elution Buffer heated to 65°C. The DNA collected is stored at -20°C.
A total of 5 PCR targets were included and 5 µl of DNA was used in each real-time PCR. Real-time PCR reactions were performed using total volumes of 20 µl containing 10 µl of master mix (Quantitect; Qiagen), 0.5 µL of each primer (20 µM), 2 µL of probes (3 µM), 2 µL of distilled water and 5 µL of template DNA (Table 1). The analyses were carried out using a CFX96™ Real-Time PCR detection test (Bio-Rad Life Science, Marnes-la-Coquette, France). Amplification reactions were performed as follows: 95°C for 15 minutes followed by 44 cycles of 60°C for 0.5 minutes and 72°C for 1 minute. Negative and positive control samples were included in each PCR run. The PCR output from this system consisted of a Cycle Threshold value (Ct), representing the amplification cycle in which the level of the fluorescent signal exceeded the background fluorescence. Therefore, low Ct values correspond to high parasite-specific DNA loads in the test sample, and vice versa. The maximum Ct value was set at 37.
| Parasites | Name | Primes/Probes | Target region | Reference |
|---|---|---|---|---|
| Protozoa | ||||
| Giardia lamblia (intestinalis or duodenalis) | Giardia-80F | 5’-GACGGCTCAGGACAACGGTT-3’ | 18s | [18] |
| Giardia-127R | 5’-TTGCCAGCGGTGTCCG-3’ | |||
| Giardia-105T | 5’-FAM-CCCGCGGCGGTCCCTGCTAG-TAMRA-3’ | |||
| Helminths | ||||
| Ascaris lumbricoides | Alum96F | 5’-GTAATAGCAGTCGGCGGTTTCTT-3’ | ITS-1 | [19] |
| Alum183R | 5’-GCCCAACATGCCACCTATTC-3’ | |||
| Alum124T | 5’-FAM-TTGGCGGACAATTGCATGCGAT-TAMRA-3’ | |||
| Strongyloides stercoralis | Stro-1530F | 5’-GAATTCCAAGTAAACGTAAGTCATTAGC-3’ | 18s | [20] |
| Stro-1630R | 5’-TGCCTCTGGATATTGCTCAGTTC-3’ | |||
| Stro-1586T | 5’FAM-ACACACCGGCCGTCGCTGC-TAMRA-3’ | |||
| Trichuris trichiura | TrichF | 5’-TTGAAACGACTTGCTCATCAACTT-3’ | 18s | [21] |
| TrichR | 5’-CTGATTCTCCGTTAACCGTTGTC-3’ | |||
| TrichP | 5’-FAM-CGATGGTACGCTACGTGCTTACCATGG-TAMRA-3’ | |||
Table 1: List of primers and probes (Applied Biosystems UK) used in this study for the detection of 5 intestinal parasites by real-time PCR.
Statistical analysis
Data were entered on Excel and analysis performed using Stata 13. Variables were compared using Fisher Exact test Chi square depending on the conditions of application of these tests were estimated to assess the performance of microscopy and RT-PCR for the detection of parasitic infections. Agreement of the two methods was assessed using Cohen’s Kappa test.
Ethics statement
This study was conducted according to the declaration of Helsinki and existing national legal and regulatory requirements. The protocol was reviewed and approved by the Senegalese Ethics Committee at the Cheikh Anta Diop University. Approval number 0258/2017/CER/UCAD. Informed consent of parent or legal representative was required prior to the participation in the study. To respect confidentiality, an identification code was given to each participant.
Results
Socio demographic characteristics of the study population
In total, 253 people participated in this study, 221 (87.3%) boys and 32 (12.65%) girls (age group=4 to 21 years, mean age=11.43 years). Participants were stratified by age and sex. There were 105 (41.5%) participants aged 1 years to 10 years, 148 (58.5%) aged 11 years to 21 years. A total of 44 people (17.39%) had a positive result by microscopy and 94 or 37.15% had a positive result by RT-PCR (Table 2).
| Number | Percentage | 95% IC | |
|---|---|---|---|
| Age | |||
| ≤ 10 | 105 | 41.50% | [35.3-47.8] |
| ˃10 | 148 | 58.50% | [52.1-64.6] |
| Gender | |||
| Male | 221 | 87.35% | [82.6-91.1] |
| Female | 32 | 12.65% | [8.8-17.3] |
| Microscopic result | |||
| Positive | 44 | 17.39% | [12.9-22.6] |
| Negative | 209 | 82.38% | [80.4-89.4] |
| Real-time PCR result | |||
| Positive | 94 | 37. 15 % | [31.3-43.5] |
| Negative | 159 | 62.45% | [56.4-68] |
Table 2: Baseline characteristics of the study population.
Direct comparisons between diagnostic techniques on individual samples from all participants were made using p-value and Kappa agreement statistics where applicable (Table 3). The results show a good, moderate and fair match for Ascaris. For Giardia and Trichuris, respectively, the difference in detection was statistically significant (p=0.0009, p=0.003). For all parasites analysed, the RTPCR identified a large number of positive samples not detected by microscopy (25 Ascaris, 11 Strongyloides, 47 Giardia and 12 Trichirus), while a small number of positive samples by microscopy were not identified as positive by RT-PCR (7 Ascaris, 5 Giardia and 3 Trichirus).
| PCR | Microscopy | Kappa | p | ||
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Ascaris | Positive | 23 | 25 | 0.51 | 0 |
| Negative | 7 | 198 | |||
| Strongyloide | Positive | 0 | 11 | 0 | - |
| Negative | 0 | 242 | |||
| Trichuris | Positive | 2 | 12 | 0.18 | 0.003 |
| Negative | 3 | 236 | |||
| Giardia | Positive | 6 | 41 | 0.14 | 0.0009 |
| Negative | 5 | 200 | |||
Table 3: RT-PCR and microscopy parasite prevalence agreement statistics.
Microscopy detected an association of Ascaris and Trichocephalosis infection. Most of the coinfections were detected by RT-PCR which was able to detect both double and triple parasitisms, the association between helminth (Ascaris and Trichocephalosis) but also between helminth and protozoan (Strongyloidosis+Giardia). The association between helminth and protozoan could be detected at 9.09% (Table 4).
| Number | |
|---|---|
| Microscopic examination | |
| Ascaris+Trichocephalose | 1 |
| Real Time PCR | |
| Ascaris+Trichocephalose | 5 |
| Ascaris+Strongyloide | 6 |
| Ascaris+Giardia | 12 |
| Strongyloide+Trichoceohalose | 1 |
| Trichocephalose+Giardia | 2 |
| Strongyloide+Giardia | 7 |
| Ascaris Strongyloide Giardia | 4 |
| Ascaris Strongyloide Trichocephalose Giardia | 1 |
| Ascaris Strongyloide Giardia | 1 |
Table 4: Presentation of coinfections identified by microscopic examination and real-time PCR assays.
Comparison of the direct examination and the ritchie concentration
Diagnostic sensitivity was estimated for the direct examination and the Ritchie concentration method and for each of the parasite species (Figure 1). The direct examination and the Ritchie method have some sensitivity in detecting every helminth, with the exception of Strongyloides stercoralis. The direct smear was inferior to the Ritchie concentration method for the detection of Trichirus trichiura and Ascaris and Giardia intestinalis. Likewise, the direct smear was inferior to the Ritchie method for the detection of G. intestinalis.
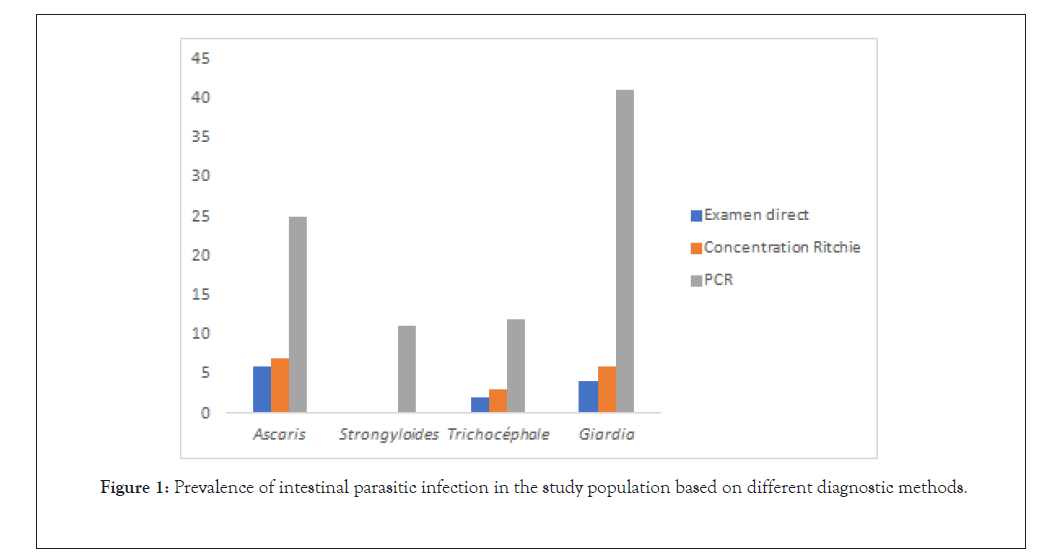
Figure 1: Prevalence of intestinal parasitic infection in the study population based on different diagnostic methods.
Microscopy versus PCR
The sensitivity of PCR for the detection of each of the parasite species tested was greater than that of any of the microscopic techniques applied (Figure 1). This difference was statistically significant p(0.003) for both microscopic methods used for S. stercoralis, and G. intestinalis and for the direct smear for the detection of A. lumbricoides. For some species, the sensitivity of the microscopic technique was much lower than that of PCR. For G. intestinalis, the sensitivity of FEC was 4.45% while that of PCR was 18.83%.
Discussion
This study was initiated to get an idea on the distribution of the most common intestinal parasitoses in children with unfavourable living conditions after 4 courses of MDA in the study area [17- 20]. However, the diagnostic methods used in prevalence surveys rely on microscopy techniques. The first objective of this study was to obtain an overview of certain intestinal helminths and protozoan infections (Giardia intestinalis). The levels of intestinal parasitic infections observed in our study from microscopy are close to the results of other previous studies carried out in rural areas (Keur Socé) by Tine and al which showed 26.2% prevalence for intestinal parasites with a predominance of protozoan parasites [21]. Compared to the urban area in Senegal, similar results showed a prevalence of 26.8% while Ndiaye reported a prevalence of 20.3% [22,23].Similar results are observed in some African countries, namely Kenya (25.6%) and Ethiopia (26.2%) [24,25]. The high positivity rate in our study observed with real-time PCR tests compared to microscopy confirms the results of previous studies showing the superiority of real-time PCR in the detection of intestinal parasites [26,27]. In addition, a similar molecular study recently conducted in the United States, using stool samples from western Kenya, demonstrated that certain parasites (Ascaris lumbricoides and N. americanus) can be detected by real-time PCR with a high rate of sensitivity (98% for both parasites) compared to microscopy (70% and 32%, respectively) [28].
Our results showed a relatively high rate of coinfections detected by real-time PCR compared to microscopy. This finding is interesting because polyparasitism is an important factor in the selection process of antiparasitic drugs for mass drug administration.
In the diagnosis of helminths, both methods were able to easily detect A. lumbricoides and Trichuris trichiura with little difference observed in terms of sensitivity, as the diagnosis appears simple (except at low concentration) compared to difficult microscopic detection of species of protozoa [29]. However, the real-time PCR test could only detect S. stercoralis species. This result could be explained by the fact that no specific concentration technique was used for the identification of Strongyloides larvae in our study. Indeed, the diagnosis of S. stercoralis infection is difficult due to the small number of ova and larvae available in the faeces [29]. Therefore, several stool examinations must be performed and sometimes specific concentration techniques, such as the Baermann method, are necessary to increase the rate of sensitivity. Unfortunately, these methods are not always used in routine diagnosis and are not available in surveys, leading to underestimation of infections during epidemiological surveys. Thus, real-time PCR testing appears to be an appropriate method to assess the burden of parasitic infections and the effectiveness of ongoing mass drug distribution programmes in the field during epidemiological surveys.
It is widely recognized that PCR could be particularly useful for the detection of Intestinal parasites in areas of low transmission and in the post-control settings. The co-infection between helminths and protozoa observed in this study highlighted the need to target certain parasites such as G. intestinalis in mass drug administration programmes, which are currently against soil-transmitted helminths.
Conclusion
The results showed that intestinal parasites, especially helminths, are still present despite MDA in children. However, it is difficult to achieve high diagnostic sensitivity for all species. Conventional techniques such as the Ritchie Modified Concentration Technique are useful for the detection of certain species of helminths, but they lack sensitivity for certain species of parasites. RT-PCR can detect intestinal parasites and polyparasitism more accurately, but is generally not feasible in countries with limited resources, at least not in peripheral laboratories. The real advantage of RT-PCR lies in its ability to more accurately determine the intensity of infection and the potential to report the results in more understandable “quantitative” terms, which will prove inherently more useful in determining the infection. Success of therapeutic and intervention trials. Therefore, it is necessary to adopt a more sensitive approach in the field for the diagnosis of parasitic infections in order to hopefully interrupt the chain of transmission of intestinal parasitosis.
Acknowledgment
We acknowledge the heads of villages, families and the staff of Guédiawaye health post for their diligent help during this study. We also thank the patient for their participation and cooperation.
Funding
Field survey and stools collection were supported by the departement of Parasitology –Mycology.
REFERENCES
- Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009;373(9674):1570-1575.
- Prevention and control of schistosomiasis and soil-transmitted helminthiasis: WHO technical report series N° 912. World Health Organization. 2002.
- Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7(1):37.
- Alum A, Rubino JR, Ijaz MK. The global war against intestinal parasites--should we use a holistic approach?. Int J Infect Dis. 2010;14(9):e732-e738.
- Okyay P, Ertug S, Gultekin B, Onen O, Beser E. Intestinal parasites prevalence and related factors in school children, a western city sample-Turkey. BMC Public Health. 2004;4(2):64.
- Amuta E, Olusi T, Houmsou R. Relationship of intestinal parasitic infections and malnutrition among school children in Makurdi, Benue State, Nigeria. Internet J Epidemiol. 2013;7(1):20-24.
- Levecke B, Montresor A, Albonico M, Ame SM, Behnke JM, Bethony JM, et al. Assessment of anthelmintic efficacy of mebendazole in school children in six countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis. 2014;8(10):e3204.
- Gabrielli AF, Montresor A, Chitsulo L, Engels D, Savioli L. Preventive chemotherapy in human helminthiasis: theoretical and operational aspects. Trans R Soc Trop Med Hyg. 2011;105(12):683-693.
- Allen AV, Ridley DS. Further observations on the formol-ether concentration technique for faecal parasites. J Clin Pathol. 1970;23(6):545–546.
- Becker SL, Lohourignon LK, Speich B, Rinaldi L, Knopp S, N'goran EK. Comparison of the Flotac-400 dual technique and the formalin-ether concentration technique for diagnosis of human intestinal protozoon infection. J Clin Microbiol. 2011;49(6):2183-2190.
- Saugar JM, Merino FJ, Martín-Rabadán P, Fernández-Soto P, Ortega S, Gárate T, et al. Application of real-time PCR for the detection of Strongyloides spp. in clinical samples in a reference center in Spain. Acta Trop. 2015;142(1):20-25.
- Nazeer JT, Khalifa KES, von Thien H, El-Sibaei MM, Abdel-Hamid MY, Tawfik RAS, et al. Use of multiplex real-time PCR for detection of common diarrhea causing protozoan parasites in Egypt. Parasitol Res. 2013;112(2):595-601.
- Verweij JJ. Application of PCR-based methods for diagnosis of intestinal parasitic infections in the clinical laboratory. Parasitology. 2014;141(14):1863-1872.
- Verweij JJ, Stensvold CR. Molecular testing for clinical diagnosis and epidemiological investigations of intestinal parasitic infections. Clin Microbiol Rev. 2014;27(2):371–418.
- Mejia R, Vicuña Y, Broncano N, Sandoval C, Vaca M, Chico M, et al. A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations. Am J Trop Med Hyg. 2013;88(6):1041-1047.
- Durvasula K, Butts T. High throughput solution for DNA extraction from stool samples using magnetic beads. Omega Bio-tek. 2004.
- Verweij JJ, Blangé RA, Templeton K, Schinkel J, Brienen EAT, van Rooyen MAA. Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real-time PCR. J Clin Microbiol. 2004;42(3):1220-1223.
- Wiria AE, Prasetyani MA, Hamid F, Wammes LJ, Lell B, Ariawan I, et al. Does treatment of intestinal helminth infections influence malaria? Background and methodology of a longitudinal study of clinical, parasitological and immunological parameters in Nangapanda, Flores, Indonesia (ImmunoSPIN Study). BMC Infect Dis. 2010;10(1):77.
- Verweij JJ, Canales M, Polman K, Ziem J, Brienen EAT, Polderman AM, et al. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg. 2009 Apr;103(4):342-346.
- Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, et al. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J Clin Microbiol. 2013;51(2):472-480.
- Tine RCK, Faye B, Ndour CT, Sylla K, Sow D, Ndiaye M, et al. Parasitic infections among children under five years in Senegal: Prevalence and effect on anaemia and nutritional status. ISRN Parasitol. 2013;2013(1):272701.
- K Sylla, RCK Tine, D Sow, T Dieng, B Faye. Epidemiological aspects of intestinal parasitic infection diagnosed in parasitology and mycology laboratory of Fann hospital, Dakar. Med Afr Noire. 2013;60(1):339-346.
- Ndiaye D, Ndiaye M, Gueye PAL, Badiane A, Fall ID, Ndiaye YD, et al. Intestinal helminthiasis diagnosed in Dakar, Senegal. Med Sante Trop. 2013;23(1):35-38.
- Mbae CK, Nokes DJ, Mulinge E, Nyambura J, Waruru A, Kariuki S. Intestinal parasitic infections in children presenting with diarrhoea in outpatient and inpatient settings in an informal settlement of Nairobi, Kenya. BMC Infect Dis. 2013;13(1):243.
- Tulu B, Taye S, Amsalu E. Prevalence and its associated risk factors of intestinal parasitic infections among Yadot primary school children of South Eastern Ethiopia: a cross-sectional study. BMC Res Notes. 2014;7(2):848.
- Easton AV, Oliveira RG, O'Connell EM, Kepha S, Mwandawiro CS, Njenga SM, et al. Multi-parallel qPCR provides increased sensitivity and diagnostic breadth for gastrointestinal parasites of humans: field-based inferences on the impact of mass deworming. Parasit Vectors. 2016;9(1):38.
- Hotez P. Enlarging the “audacious goal”: elimination of the world’s high prevalence neglected tropical diseases. Vaccine. 2011;29(4):D104–D110.
- Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7(1):e2002.
- Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, et al. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis. 2008;2(11):e331.
Citation: Lelo S, Ly F, Lam A, Fall CB, Manga I, Tairou F, et al. (2021) Detection of Intestinal Parasites in Stool Samples by Microscopy and Real-Time PCR in Children with Vulnerable Living Conditions in Dakar, Senegal. J Bacteriol Parasitol. 12: 398.
Copyright: © 2021 Lelo S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

