Indexed In
- Open J Gate
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Mini Review - (2023) Volume 11, Issue 4
Design, Synthesis, Characterization and Antimicrobial Activity of Oxothiazolidin Derivatives
Foram J Patel, Kinjal D Patel, Vipul B Audichya* and Rashmikant A PatelReceived: 07-Jul-2023, Manuscript No. MCA-23-22082; Editor assigned: 10-Jul-2023, Pre QC No. MCA-23-22082 (PQ); Reviewed: 25-Jul-2023, QC No. MCA-23-22082; Revised: 01-Aug-2023, Manuscript No. MCA-23-22082 (R); Published: 09-Aug-2023, DOI: 10.35248/2329-6798.23.11.424
Abstract
The basic compound N-[2-(substituted phenyl)-4-oxo-1,3-thiazolidin-3-yl]-4-chloro-3-nitrobenzamide (2 a-j) have been synthesized by reaction of N’-[(substituted phenyl)methylidene]-4-chloro-3-nitrobenzohydrazide (1a-j) and aromatic aldehyde in presence of benzene, further react with thioglycolic acid.
The structural assignment of the compounds was based on elements analysis and Infrared Spectroscopy (IR), 1H Nuclear Magnetic Resonance (NMR), 13C Nuclear Magnetic Resonance (NMR) spectral data. All the synthesized compounds have been screened for their antimicrobial activity to gram-positive and gram-negative bacterial strains and antifungal activity. The antimicrobial activities of the synthesized compounds have been compared with standard antibiotic drugs like Gentamycin and K.Nystatin. Purity of synthesized compounds has been checked by Thin Layer Chromatography.
Keywords
Schiff base; Thiazolidinone; Anti-microbial activity
Introduction
Thiazolidinones, which belong to an important group of heterocyclic compounds, have been extensively explored for their application in the field of medicine. Numerous reports have been appeared in the literature which highlights their chemistry and use. Cylcoadition reactions of schiff bases with mercapto acetic acid results into the formation of thiazolidinones. Litrature survey reveales several substituted biologically active thiazolidinones compounds were prepared and found to have antibacterial and antifungal properties [1-7]. It has also been found to possess anti tubercular activity as well as anticancer, anti- inflammatory activity, analgesic, antioxidant, anti-viral, anti-HIV, anti-malarial activity [8-17]. Cu catalyzed synthesis of pyrrole bearing novel isoxazole derivatives via [3+2] cycloaddition reaction [18]. As a part of the surge of interest in heterocycles that have been explored for developing pharmaceutically important molecule, 1-acetyl-3,-5- diarylpyrazolines have played an important role in medicinal chemistry as they possess wide range of therapeutic activities. The preparation of novel thiazolidinone derivatives of type (2.1a-j) have been under taken by reaction of schiff base of type (1a-j) with thioglycolic acid in dry benzene.
Literature Review
Antimicrobial activity
The Minimal Inhibitor Concentration method (MIC) of synthesized compound was obtained against two representatives Gram positive organisms viz. S.aureus (MTCC 96), S.pyogenus (MTCC 442) and two Gram-negative organisms viz. E.coli (MTCC 443), P.aeruginosa (MTCC 1688) and evaluated for MIC against fungal strains C.albicans at a concentration of 6.25 µg/ml.by the broth dilution method recommended by National Committee for Clinical laboratory (NCCL) standards [19,20] and Dimethyl Sulfoxide (DMSO) was used as a solvent. The MIC values of synthesized compounds were compared with standard antibiotic drugs like Gentamycin and K.Nystatin. The minimal inhibitor concentrations (MIC) of synthesized compounds are represented in Table 1.
| Minimal Inhibition Concentrations (MIC) of bacterial strains in µg/ml |
Minimal Inhibition Concentrations (MIC) of fungal strains in µg/ml |
|||||
|---|---|---|---|---|---|---|
| Gram positive strains | Gram negative strains |
|||||
| Sr. No. | R1 | S. aureus MTCC 96 |
S. pyogenes MTCC 442 |
E. coli MTCC 443 |
P. aeruginosa MTCC 1688 |
C. albicans MTCC 227 |
| 2a | 2-hydroxy-5-bromo | 500 | 250 | 500 | 500 | 125 |
| 2b | 2-methoxy-5-bromo | 250 | 100 | 100 | 500 | 1000 |
| 2c | 2-ethoxy-5-bromo | 500 | 50 | 50 | 500 | 1000 |
| 2d | 3-bromo-4-hydroxy | 100 | 100 | 500 | 100 | 500 |
| 2e | 3-bromo-4-methoxy | 500 | 100 | 500 | 500 | 125 |
| 2f | 3-bromo-4-ethoxy | 100 | 25 | 25 | 50 | 1000 |
| 2g | 3, 5 dibromo-4-hydroxy | 25 | 25 | 250 | 250 | 1000 |
| 2h | 5-bromo-3, 4 dimethoxy | 100 | 50 | 50 | 500 | 100 |
| 2i | 3-ethoxy-4-hydroxy-5-bromo | 250 | 50 | 6.25 | 12.5 | 1000 |
| 2j | 5-bromo-3, 4 diethoxy | 100 | 100 | 12.5 | 12.5 | 250 |
| Std. Drug |
Gentamycin | 0.25 | 0.5 | 0.05 | 1.0 | - |
| K.Nystatin | - | - | - | - | 100 | |
Table 1: Antibacterial and antifungal activities of N-[2-(3-bromo-4-ethoxyphenyl)-4-oxo-1, 3-thiazolidin-3-yl]-4-chloro-3-nitrobenzamide.
Experimental analysis
All the melting points were measured by open capillary method and are uncorrected. The IR absorption spectra (v max in cm-1) were recorded on a Shimadzu FTIR 8400 Spectrophotometer, 1H NMR (δ ppm) and 13C NMR spectra were recorded on a BRUKER (300 MHz) Spectrometer using Tetramethylsilane (TMS) as internal standard (Figures 1-3).
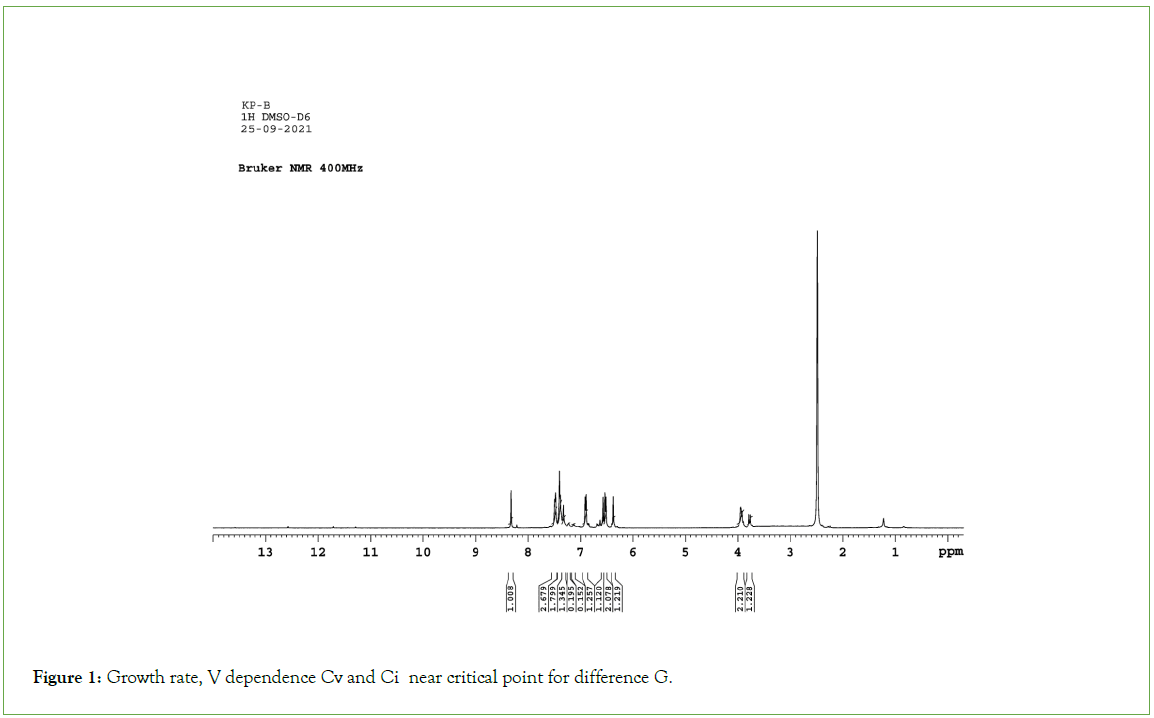
Figure 1: Growth rate, V dependence Cv and Ci near critical point for difference G.
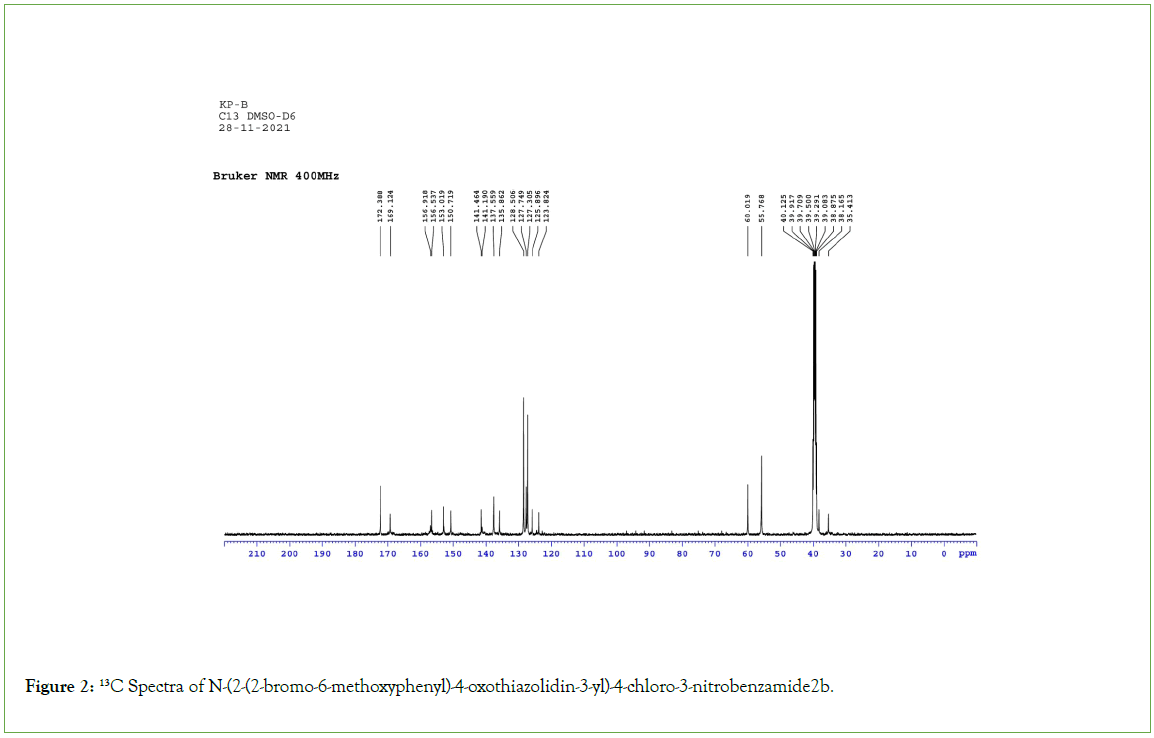
Figure 2: 13C Spectra of N-(2-(2-bromo-6-methoxyphenyl)-4-oxothiazolidin-3-yl)-4-chloro-3-nitrobenzamide2b.
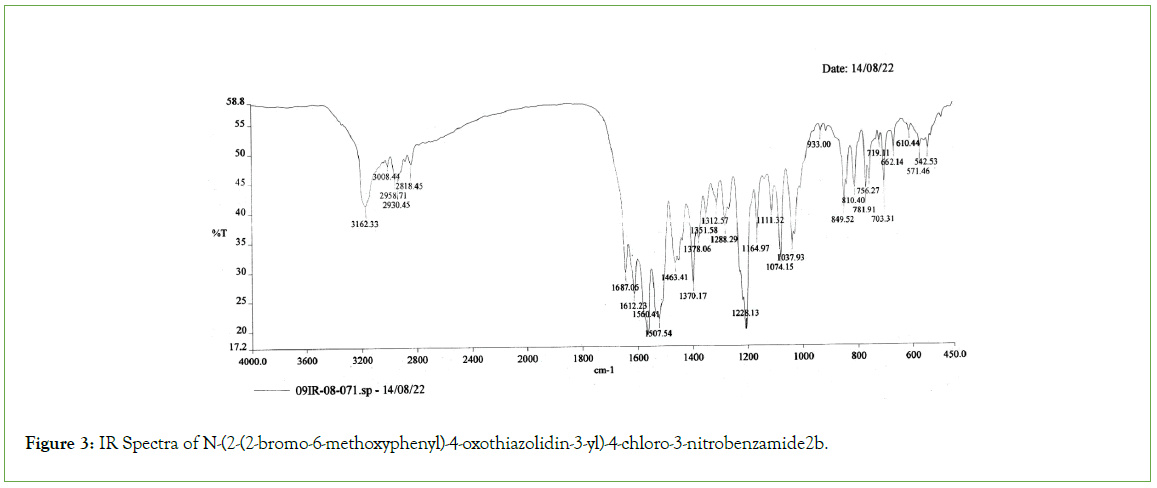
Figure 3: IR Spectra of N-(2-(2-bromo-6-methoxyphenyl)-4-oxothiazolidin-3-yl)-4-chloro-3-nitrobenzamide2b.
General preparation of N-(2-(2-bromo-6-hydroxyphenyl)-4-oxothiazolidin-3-yl)-4-chloro-3-nitrobenzamide2a,(2 a-j)
A mixture of Schiff base (0.01 mole) and thioglycolic acid (0.015 mole) were dissolved in dry benzene (50 ml) and Dimethylformamide (DMF) (20 ml) were refluxed for 20-22 hours. Completion of the reaction was checked by TLC. Benzene was distilled out and then reaction mixture was cooled and poured in to ice cold water and stirred for 2 hrs. Further treated with 20% sodium bicarbonate solution, the product separated was filtered and washed with water and crystallized from ethanol (95%).
Similarly, other compound of this series (2 a-j) have been prepared. The physical constants are recorded below:
N-(2-(2-bromo-6-hydroxyphenyl)-4-oxothiazolidin-3-yl)-4-chloro-3-nitro benzamide 2a:
White Creamish yellow solid,
Yield: 70%:
mp: 190-195°C;
Reaction time: 24 hrs:
Rf value: 0.45;
Molecular Weight: 472.69;
Anal. Calcd. for C16H11BrClN3O5S: C-40.65, H-2.35, X- 24.43, N-1.19, S-6.78;
Found: C-40.63, H-2.32, X- 24.44, N-1.21, S-6.80%.
IR (KBr) cm-1 2a: 1662 (C=O str, thiazolidinone), 1178 (C-N str, thiazolidinone), 732 (C-S-C str., thiazolidinone), 1628(S-C=N str., thiazolidinone), 1511(NO2str. (asym.)), 1389 (NO2str. (sym.)) 1242 (C-O-C (sym)), 1058(C-O-C (asym)), 3321 (-NHstr.), 718 (C-Br str.), 604 (C-Cl str.).
N-(2-(2-bromo-6-methoxyphenyl)-4-oxothiazolidin-3-yl)-4-chloro-3-nitro benzamide2b:
Light Creamish Yellow Solid,
Yield: 73%:
mp: 255-260°C;
Reaction time: 22 hrs:
Rf value: 0.37;
Molecular Weight: 486.72;
Anal. Calcd. for C17H13BrClN3O5S: C-41.95, H-2.69, X- 23.70, N-8.63, S-6.59;
Found: C-41.96, H-2.71, X- 23.74, N-8.60, S-6.61%;
N-(2-(2-bromo-6-ethoxyphenyl)-4-oxothiazolidin-3-yl)-4- chloro-3-nitro benzamide2c:
Yellow Creamish solid,
Yield: 68%:
mp: 240-245°C;
Reaction time: 21hrs:
Rf value: 0.53;
Molecular Weight: 500.75;
Anal. Calcd. for C18H15BrClN3O5S: C-42.17, H-3.02, X- 23.04, N-8.39, S-6.40;
Found: C-42.19, H-3.05, X- 23.14, N-8.42, S-6.22%;
N-(2-(3-bromo-4-hydroxyphenyl)-4-oxothiazolidin-3-yl)-4- chloro-3-nitro benzamide2d:
White Creamish solid,
Yield: 78%:
mp: 155-160°C;
Reaction time: 20 hrs:
Rf value: 0.32;
Molecular Weight: 472.69;
Anal. Calcd. for C16H11BrClN3O5S: C-40.65, H-2.35, X- 24.43, N-1.19, S-6.78;
Found: C-40.68, H-2.31, X- 24.44, N-1.16, S-6.77%.
1H NMR (δ ppm) 2d: 3.771-3.781 (d, 1H, CH2 (thiazolidinone)), 3.914-3.993 (d, 1H, CH2(thiazolidinone), 6.37 (Singlet, 1H, N-CH-S thiazolidinone)), 8.367 (Singlet, 1H, N-H), 6.516 (s, 1H, OH merged with Ar-H), 6.536-7.490 (Multiplet, 6H, Ar-H).
N-(2-(3-bromo-4-methoxyphenyl)-4-oxothiazolidin-3-yl)-4- chloro-3-nitro benzamide2e:
Light white yellow solid,
Yield: 67%:
mp: 225°C-230°C;
Reaction time: 20 hrs:
Rf value: 0.54;
Molecular Weight: 486.72;
Anal. Calcd. for C17H13BrClN3O5S: C-41.95, H-2.69, X- 23.70, N-8.63, S-6.59;
Found: C-41.93, H-2.73, X- 23.71, N-8.66, S-6.62%.
13C NMR (CDCl3) δppm 2e: 150.72( C-1), 135.86( C-2), 169.123( C-3, CO-CH2-S), 172.388( C-4, CO-CH2-S), 35.413( C-5, COCH 2-S), 60.018( C-6, N-CH-S), 141.19( C-7), 55.76( C-8,-OCH3), 153.019( C-9).
N-(2-(3-bromo-4-ethoxyphenyl)-4-oxothiazolidin-3-yl)-4- chloro-3-nitro benzamide2f:
Yellow Creamish solid,
Yield: 70%:
mp: 195°C-200°C;
Reaction time: 22 hrs:
Rf value: 0.56;
Molecular Weight: 500.75;
Anal. Calcd. for C18H15BrClN3O5S: C-42.17, H-3.02, X- 23.04, N-8.39, S-6.40;
Found: C-42.19, H-3.07, X- 23.11, N-8.44, S-6.42%.
4 -chloro-N- ( 2 - (3,5-dibromo- 4 -hydroxypheny l ) -4 oxothiazolidin-3-yl)-3-nitro benzamide2g:
Yellow Creamish solid,
Yield: 60%:
mp: 135°C-240°C;
Reaction time: 19 hrs:
Rf value: 0.55;
Molecular Weight: 551.59;
Anal. Calcd. for C16H10Br2ClN3O5S: C-34.84, H-1.83, X- 35.40, N-7.62, S-5.81;
Found: C-34.86, H-1.82, X- 35.45, N-7.60, S-5.80%;
N-(2-(3-bromo-4,5-dimethoxyphenyl)-4-oxothiazolidin-3-yl)- 4-chloro-3-nitro benzamide2h:
White Creamish yellow solid,
Yield: 65%:
mp: 265°C-270°C;
Reaction time: 22 hrs:
Rf value: 0.61;
Molecular Weight: 516.75;
Anal. Calcd. for C18H15BrClN3O6S: C-41.84, H-2.93, X- 22.32, N-8.13, S-6.21;
Found: C-41.82, H-2.91, X- 22.30, N-8.11, S-6.24%;
N-(2-(3-bromo-5-ethoxy-4-hydroxyphenyl)-4-oxothiazolidin- 3-yl)-4-chloro-3-nitrobenzamide2i:
Yellow Creamish solid,
Yield: 69%:
mp: 210°C-215°C;
Reaction time: 21 hrs:
Rf value: 0.38;
Molecular Weight: 516.75;
Anal. Calcd. for C18H15BrClN3O6S: C-41.84, H-2.93, X- 22.32, N-8.13, S-6.21;
Found: C-41.86, H-2.94, X- 22.30, N-8.10, S-6.20%;
N-(2-(3-bromo-4,5-diethoxyphenyl)-4-oxothiazolidin-3-yl)-4- chloro-3-nitro benzamide2j:
Light Creamish yellow solid,
Yield: 72%:
mp: 120-125°C;
Reaction time: 24 hrs:
Rf value: 0.0.39;
Molecular Weight: 544.80;
Anal. Calcd. for C20H19BrClN3O6S: C-44.09, H-3.52, X- 21.18, N-7.71, S-5.89;
Found: C-44.11, H-3.53, X- 21.22, N-7.74, S-5.91%;
Reaction Scheme
Step-1: Synthesis of (E)-N’-(2-bromo-6-hydroxybenzylidene)-4- chloro-3-nitrobenzohydrazide (1a-j):
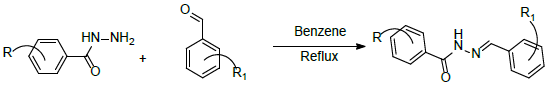
Step-2: N-(2-(2-bromo-6-hydroxyphenyl)-4-oxothiazolidin-3-yl)-4- chloro-3-nitro benzamide (2a-j).
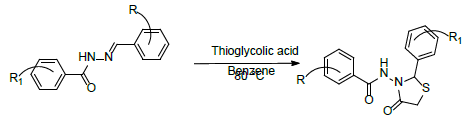
• Where, R=3-nitro4-chloro
• Where, R1= 2-hydroxy-5-bromo, 2-methoxy-5-bromo,2-ethoxy- 5-bromo,3-bromo-4-hydroxy,3-bromo-4-methoxy,3-bromo-4- ethoxy,3, 5 dibromo-4-hydroxy,5-bromo-3, 4 dimethoxy,3- ethoxy-4-hydroxy-5-bromo,5-bromo-3, 4 diethoxy.
Spectral result and discussion
Thiazolidinone ring was characterized by several absorption peaks in the infrared spectrum. Spectra of (2a) showed absorption at 1628 cm-1, which is a characteristics of S-C=N. It was also observed the stretching band of C-S-C of thiazolidinone ring at 732 cm-1. C-N stretching band of thiazolidinone is exhibited 1178 cm-1. Sharp absorption band appearing at 1662 cm-1 was attributed to carbonyl function of C=O. The band of secondary amine –NH observed at 3321 cm-1.The symmetric and asymmetric bands of nitro group observed at 1389 cm-1and 1511 cm-1. C-Cl str. and C-Br str. band observed at 604 cm-1 and 718 cm-1 respectively. 1H NMR spectra of (2d) under study observed that magnetically non equivalent protones of thiazolidinone ring (–CH2) attributed as two doublets at 3.771δppm-3.781 δppm and 3.914δppm-3.993δppm. Both the protons are well separated from each other, one hydrogen atom of each pair being above the phenyl ring in the shielding cone and the other one, outside of it. The diastereotopic hydrogen atoms are well separated. Singlet of-OH group appeared at 6.51 δppm which is merged with aromatic protons. The sharp singlet appeared at 6.37 δppm indicated presence of N-CH-S group of thiazolidinone nucleus. While the proton of secondary amine exhibited as sharp singlet at 8.36 δppm. In aromatic region, protons resonated in the range of, 6.536δ ppm-7.490 δppm. In 13CNMR spectra of the compound (2e) carbons of the thiazolidinone were resonated at 60.018 δppm (C-10, N-CH-S). Ketone of thiazolidinone showed at 172.38 δppm (C-7, CO-CH2-S). Carbon appeared at 35.41δppm confirms the presence of (C-9, CO-CH2-S). The signal appeared at 55.76δppm (C-17, OCH3) was assign the presence of methoxyl carbon.
Biological result and discussion
The observation of inhibition data suggest that the only two compounds 2(i) and 2(j) have displayed excellent activity against gram negative bacterial strains E.coli and P.aeruginosa respectively. In addition compound 2(g) have exhibited good activity against both gram positive bacterial strains. Compound 2(f) have shown good activity against gram negative bacterial strain E.coli and gram positive bacterial strain S.pyogenes respectively. Compound 2h showed moderate activity against both gram negative strains. Compound 2(h) exhibited moderate activity against gram negative bacterial strain P.aeruginosa and gram positive bacterial S.pyogenes.
The MIC values of screened compounds suggest that the test compound 2(h) showed excellent activity against fungal strains comparable to reference agents K Nystatin. Compound 2(e) have showed good activity against the tested organisms. While the other compounds showed poor or no activity even at concentration of 200 µg/ml. The overall results of anti-bacterial and antifungal activity are mentioned in Table 1.
Conclusion
In conclusion, we have synthesized few novel hybrid molecules N-(2- (2-bromo-6-hydroxyphenyl)-4-oxothiazolidin-3-yl)-4-chloro-3-nitro benzamide (2a-j) neat reaction in microwave and also in presence of benzene. Only two compounds 2i and 2j have displayed excellent activity against gram negative bacterial strains and P.aeruginosa respectively. In addition compound 2g have exhibited good activity against both gram positive bacterial strains. Compound 2f have shown good activity against gram negative bacterial strain E.coliand gram positive bacterial strain S.pyogenes respectively. Compound 2c showed moderate activity against both gram negative strains. Compound 2h exhibited moderate activity against gram negative bacterial strain P.aeruginosa and gram positive bacterial S.pyogenes. The test compound 2h showed excellent activity against fungal strains comparable to reference agents K Nystatin. Compound 2e have showed good activity against the tested organisms. While the other compounds showed poor or no activity even at concentration of 200 µg/ml. Research outcome of this study can be helpful for preparing new anti-bacterial molecules that is effective in numerous therapy for different Schiff base compounds.
Acknowledgement
Author also thankful to Centre of Excellence (CoE), Saurashtra University, providing facilities and Rajkot for providing analytical data. The authors additionally thank the authorities of Municipal Arts and Urban Bank Science College, Mehsana, for providing research facilities.
Conflict of Interest
All authors agree for the publication. For all authors, there is no competing interests to declare.
References
- Alegaon SG, Alagawadi KR, Pawar SM, Vinod D, Rajput U. Synthesis, characterization, and biological evaluation of thiazolidine-2, 4-dione derivatives. Med Chem Res. 2014;23:987-994.
- Patel NB, Patel MD. Synthesis and evaluation of antibacterial and antifungal activities of 4-thiazolidinones and 2-azetidinones derivatives from chalcone. Med Chem Res. 2017;26:1772-1783.
- Gilani SJ, Nagarajan K, Dixit SP, Taleuzzaman M, Khan SA. Benzothiazole incorporated thiazolidin-4-ones and azetidin-2-ones derivatives: Synthesis and in vitro antimicrobial evaluation. Arab J Chem. 2016;9:S1523-1531.
- Matar SA, Talib WH, Mustafa MS, Mubarak MS, AlDamen MA. Synthesis, characterization, and antimicrobial activity of Schiff bases derived from benzaldehydes and 3, 3′-diaminodipropylamine. Arab J Chem. 2015;8(6):850-857.
- Abo-Ashour MF, Eldehna WM, George RF, Abdel-Aziz MM, Elaasser MM, Gawad NM, et al. Novel indole-thiazolidinone conjugates: Design, synthesis and whole-cell phenotypic evaluation as a novel class of antimicrobial agents. Eur J Med Chem. 2018;160:49-60.
- Zhang H, Zhang J, Qu W, Xie S, Huang L, Chen D, et al. Design, synthesis, and biological evaluation of novel thiazolidinone-containing quinoxaline-1, 4-di-N-oxides as antimycobacterial and antifungal agents. Front Chem. 2020;8:598.
- Singh I, Kumar A. Synthesis of thiazolidinone and azetidinone derivatives of benzoxazolinone as antimicrobial agents. Int J Pharm Sci Res. 2015;6(2):850-856.
- Kumar V, Shetty P, Chandra KS, Ramu R, Patil SM, Baliga A, et al. Potential fluorinated Anti‐MRSA thiazolidinone derivatives with antibacterial, antitubercular activity and molecular docking studies. Chem Biodivers. 2022;19(2):e202100532.
- Ponnuchamy S, Kanchithalaivan S, Kumar RR, Ali MA, Choon TS. Antimycobacterial evaluation of novel hybrid arylidene thiazolidine-2, 4-diones. Bioorganic & medicinal chemistry letters. 2014;24(4):1089-1093.
- Oubella A, El Mansouri AE, Fawzi M, Bimoussa A, Laamari Y, Auhmani A, et al. Thiazolidinone-linked1,2,3-triazoles with monoterpenic skeleton as new potential anticancer agents: Design, synthesis and molecular docking studies. Bioorg Med Chem Lett. 2021;115:105184.
- Ahmed NS, Alfooty KO, Khalifah SS. An efficient sonochemical synthesis of novel Schiff’s bases, thiazolidine, and pyrazolidine incorporating 1, 8-naphthyridine moiety and their cytotoxic activity against HePG2 cell lines. The Scientific World J. 2014;2014.
- Grasso S, Chimirri A, Monforte P, Fenech G, Zappalà M, Monforte AM. Compounds with potential antitumor activity. VI-2-Alkyl-3-[2-(1, 3, 4-thiadiazolyl)]-4-thiazolidinones. Il Farmaco; Edizione Scientifica. 1988;43(10):851-856.
- Hameed AA, Hassan F. Synthesis, characterization and antioxidant activity of some 4-amino-5-phenyl-4h-1, 2, 4-triazole-3-thiol derivatives. Int J Appl Sci Technol. 2014;4:202-211.
- Patil SA, Fariyike T, Marichev KO, Heras Martinez HM, Bugarin A. Medicinal applications of coumarins bearing azetidinone and thiazolidinone moieties. Future Med Chem. 2021;13(21):1907-1934.
- Nori DL, Satyanarayan KV, Avupati VR, Bugata BK, Yenupuri S. Synthesis, characterization and in vitro evaluation of some new 5-benzylidene-1, 3-thiazolidine-2, 4-dione analogs as new class of α-glucosidase inhibitors. Eur J Chem 2014;5(1):144-149.
- Subudhi B, Panda P, Kundu T, Sahoo S, Pradhan D. Synthesis and biological evaluation of some benzimidazole and thiazolidinone derivatives. J Pharm Res. 2007;6:114-118.
- Nirmal R, Meenakshi K, Shanmugapandiyan P, Prakash CR. Synthesis pharmacological evaluation of novel Schiff base analogues of 3-(4-amino) phenylimino) 5-fluoroindolin-2-one. J Young Pharm. 2010;2(2):162-168.
- Audichya VB, Savant MM, Naliapara YT. An efficient synthesis of novel isoxazole bearing pyrazole derivatives via [3+ 2] heteroannulation using cupric acetate. J Heterocycl Chem. 2022;59(2):341-350.
- Rodriguez-Tudela JL, Martinez-Suarez JV. Fluconazole and amphotericin B antifungal susceptibility testing by National Committee for Clinical Laboratory Standards broth macrodilution method compared with E-test and semiautomated broth microdilution test. J Clin Microbiol. 1997;35(1):336.
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991;83(11):757-766.
Citation: Patel FJ, Patel KD, Audichya VB, Patel RA (2023) Design, Synthesis, Characterization and Antimicrobial Activity of Oxothiazolidin Derivatives. Modern Chem Appl. 11:424.
Copyright: © 2023 Patel FJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


