Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 12, Issue 1
Depression and Anxious during COVID-19
Maya Krishnamurthy1*, NC Nagalakshmi2, Chaurasiya Raunakkumar2 and Yeshwant Kumar22Department of Pharmacology, Neelsaroj Institute of Pharmacy, Karnataka, India
Received: 03-Jan-2024, Manuscript No. HCCR-23-21106; Editor assigned: 05-Jan-2024, Pre QC No. HCCR-23-21106 (PQ); Reviewed: 19-Jan-2024, QC No. HCCR-23-21106; Revised: 26-Jan-2024, Manuscript No. HCCR-23-21106 (R); Published: 02-Feb-2024, DOI: DOI: 10.4172/2375-4273.24.12.396
Abstract
According to the COVID-19 mental disorders collaborators, major depressive disorder cases worldwide increased by 276 percent and anxiety disorder cases increased by 256 percent in 2020 as a result of the pandemic. But we argue that these prevalence estimates are probably much exaggerated. Years of trauma study have demonstrated that most people have resilience (little impact on symptoms of anxiety, depression, or both) or recovery in the wake of traumatic life experiences like bereavement or catastrophe exposure (initial short-term increase in symptoms of anxiety, or depression, or both, followed by recovery). This trend is consistent with what in-depth analyses and investigations have shown about COVID-19.
Because the authors' estimations of COVID-19's impact are based solely on studies conducted predominantly during the very early phase of the pandemic, psychological model subjects are within the framework of the collaborators' study (information series for 39 of forty-eight research passed off basically among March and May 2020; appendix). The writer extrapolated from those immediate responses to determine how SARS-CoV-2 contamination costs and human mobility would affect intellectual fitness through 2020. At that time, signs and symptoms of tension or despair were at their most extreme and may have represented an acute response to a startling event.
Keywords
COVID-19 mental disorders; SARS-CoV-2; Anxiety; Data extraction; Quality assessment
Introduction
According to the Coronavirus (COVID-19) dashboard of WHO, as of July 26, 2021, the pandemic had impacted more than 194 million people and was still expanding [1]. Numerous investigations have demonstrated that COVID-19 symptoms can linger even after an acute infection has subsided and the virus has been eliminated from the body [2]. In addition, COVID-19 sufferers, whether they had symptoms during the acute phase or not, continue to have them. The National Institute for health and Care Excellence (NICE) refer to this condition as "post- COVID-19 syndrome" and describe it as the emergence of new and/or persistent signs and symptoms for more than 12 weeks following acute respiratory syndrome [3-8]. SARS-CoV-2 (Coronavirus-2). After acute COVID-19, the majority of patients' residual symptoms and/or new symptoms disappear within 12 weeks. As of now, a stricter standard for classifying this phenomenon is provided by the NICE criteria for post-COVID-19 tsyndrome [9]. People who have been exposed to COVID-19 have a high rate of neuropsychiatric symptoms, such as fatigue and depression, which may indicate that COVID-19 has an impact on the Central Nervous System (CNS) (e.g., neurotropism of SARS-CoV-2, hyper inflammatory state and hypercoagulability following infection, especially in severe cases). Globally, depression is one of the main causes of disability (Incidence, 2017). The quality-of-life outcomes may be severely impacted by depressed symptoms and clinically significant depression in post- COVID-19 syndrome [10]. However, to our knowledge, none of the most recent assessments that looked into the neuropsychiatric effects of COVID-19 reported on the frequency of depression precisely in line with the NICE-defined post- COVID-19 syndrome. Nevertheless, research on those who survived the Severe Acute Respiratory Syndrome (SARS) pandemic in 2003 has shown that depression might persist for up to 12 months after hospital discharge. Therefore, it is possible to speculate that sadness is a common complication for those who have recovered from Coronavirus infections. Characterizing the connection between depression and post- COVID-19 syndrome is necessary when taken as a whole. The purpose of this research is to summarise the information currently available on the incidence of depression in post- COVID-19 syndrome and the risk factors linked with it [11].
Materials and Methods
A prospective registration for this review was made in PROSPERO (CRD42021254534), and the protocol was modified as the review was being written analysis [12].
The search approach carefully looked for PubMed, Ovid Medline, and Google Scholar studies
Each recognized article's title and abstract were checked to see if they met our selection criteria. Full-text publications are evaluated for eligibility after the first screening. Following separate reviews of the papers by two reviewers (OR and SE), an agreement was obtained through discussion [13]. Depression or mood problems, COVID-19, SARS-CoV-2, or Coronavirus illness 2019 were the search terms used in PubMed and Ovid Medline, along with plotted or anticipated and addition or cure or stay (*). Specifically, the terms depression, mental disease, mental health, post-COVID-19, virus, follow-up, and sequence were used to restrict the search to pertinent Google Scholar publications. Additionally, manual evaluations of reference lists from included studies and analyses of COVID-19 specific neuropsychiatric effects were performed [14].
Inclusion and exclusion criteria
Articles in English, case-control studies, cohort studies, uncontrolled observational studies, cross-sectional studies, and retrospective registry studies were all considered for inclusion in this review [15]. Reverse Transcription Polymerase Chain Reaction (RT-PCR) analysis of nasopharyngeal or saliva samples, or blood antibody testing,
• Confirmation of SARS-CoV-2 infection.
• Use of accepted standard measures (HADS-D, PHQ-9) or
criteria from the Diagnostic and Statistical Manual of Mental
Disorders (DSM-IV or DSM-V).
• Evaluation of depressive symptoms and clinically significant
depression more than 12 weeks after SARS-CoV-2 infection. If
follow-up is gauged from discharge, the infection must have
been present for 12 weeks.
Ten or more weeks after the patient is discharged from the hospital must be included if the study paper does not identify the patient's hospital stay. Animal studies, case reports, unpublished datasets, review papers, meta-analyses, the presence of a proven SARS-CoV-2 infection, depressed symptoms, and clinically significant depression, in general, were all excluded from this study. The COVID-19 pandemic's effects on society and the economy have been documented [16].
Data extraction
The following study characteristics were extracted using a standard data extraction form by two reviewers (OR and SE):
• Initial author.
• The publishing year.
• Sample size.
• A sexual analysis.
• Site.
• Median or average age.
• Study planning.
• COVID-19 symptom severity.
• Measurement of clinically significant depression and
depressive symptoms.
• Assessment time.
• Frequency of clinically significant depression and depressive
symptoms.
• Variables linked to depression.
• Other noteworthy outcomes.
Quality assessment
The papers' methodological quality was evaluated using a modified version of the Newcastle-Ottawa Scale (NOS) for prospective cohort studies. There are eight requirements, and if all of them are satisfied, there is a potential to get up to eight stars [17]. Three types of features were evaluated: The choice of research groups, the comparability of groups, and the evaluation of interest-getting results. Two items were inapplicable for uncontrolled observational studies because they lacked a control group. A NOS score of five indicates a high NOS rating, a score of three to four indicates an average score and a score of three indicates a poor NOS rating. The calibers of the included papers were evaluated separately by two reviewers (OR and SE).
Results
Systematic research result
Databases have been searched from the time of the database's inception until June 5, 2021. A total of 316 items were found in the initial search, 87 of which were determined to be duplicated. The eligibility of the remaining 229 titles and abstracts was checked [18]. 23 researches were disqualified while 30 papers satisfied the full-text evaluation criterion. Another research was chosen from the reference list of pertinent papers that satisfied the inclusion criteria. This review includes eight studies (Figure 1).
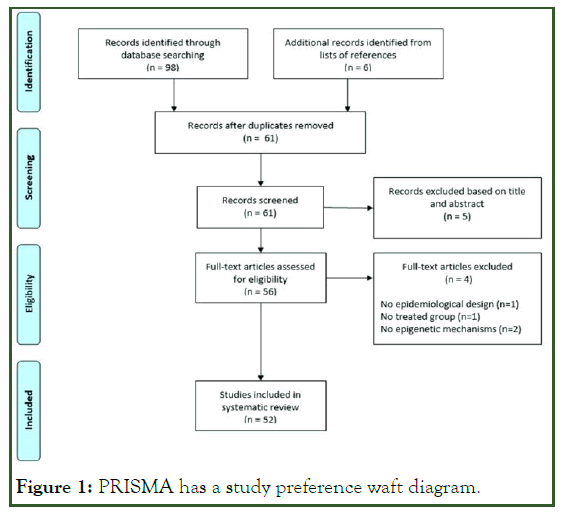
Figure 1: PRISMA has a study preference waft diagram.
Methodological excellent and hazard of bias
Three studies had a moderate NOS and five studies had a high NOS (NOS score of 5 or 6). (i.e., NOS score 4). The NOS score was 4.75 on average. Three studies had NOS values in the middle and were observational studies [19]. Two studies were penalized because depressed symptoms were not evaluated at baseline and not all patients had SARS-CoV-2 verified by RTPCR or antibody testing. Another research was penalized for not measuring depressed symptoms at the start of the trial and for not administering medication to those whose symptoms had not been checked at the follow-up.
Study traits
Six of the eight studies that were discussed employed an uncontrolled observational design and a possible cohort design. The six uncontrolled observational studies assessed the prevalence of depressive symptoms and clinically pervasive despair in post-COVID-19 syndrome, whereas the two cohort studies compared just the levels of despair between the main cohort and the control group. The DSM-V criteria (n=1) and the subsequently demonstrated scales have been used for the evaluation of despair: 13 objects Beck's Depression Inventory (BDI-13) (n=2), hospital anxiety, and there have been reports of clinically significant depression, anxiety, and depression frequency in people with post-COVID-19 syndrome [20]. Only cases of clinically severe depression and/or major depressive symptoms (defined as having a BDI-13 score below 9, a PHQ-9 score above 14, or a HADS-D score above 10) with an incidence range of 3 to 12 percent were taken into consideration. The majority of studies assessed the frequency of depressive symptoms and signs of clinically severe depression three to four months following diagnosis or release from the hospital; the frequency of symptoms was 27% and the frequency of serious depressive symptoms was 5%. Only one research found substantially higher depression ratings in COVID-19 participants (e.g., x=3 vs. x=1, on the L DASS-21 depression scale), with a control group that was not exposed to SARS CoV-2 (p=0.036). Even yet, not all patients in this research were qualified for post-COVID-19 syndrome because the assessment period in this study lasted between 12 and 215 days following diagnosis. Due to the high variability in the methods employed to measure depression and the time of the evaluation following the diagnosis of COVID-19, a meta-analysis was not carried out.
Factors related to melancholy
Gender, prior psychiatric history, psychopathology at one-month follow-up, systemic infection throughout the severe phase, age becoming the most important ability factor, and the severity of acute COVID-19 no longer being a factor, were the key factors associated with depression. In fact, during the one-month follow-up, psychopathology, girl gender, and a prior mental diagnosis had been modifiers of depression in post-COVID-19 syndrome. Intriguingly, depression persisted between the 1 and 3-month follow-up periods, despite significant improvements in PTSD symptoms, anxiety, and sleeplessness. Mazza also showed that the degree of depressed symptoms in post-COVID-19 syndrome became inversely correlated with baseline systemic infection during acute infection. Baseline measurements of the systemic immune-infection index (SII; SII=(platelets × neutrophils)/lymphocytes) classified the severity of different types of depression while examining the effects of age, gender, and hospitalization. The changes in SII also significantly exceeded expectations for changes in depression ranks. In comparison to tiny changes in SII that resulted in continuous or worse sadness rankings, lower SII among health facility admission and 3 months follow-up led to a lower melancholy severity in evaluation. Through the Consultation Multi- Expertise COVID-19 (COMEBAC) group, age was made a skilled moderator during the examination. Patients over the age of 75 were more severely impacted; 30.8 percent of them experienced severe depressive symptoms as part of post- COVID-19 syndrome, compared to 19.7 percent of patients under the age of 75. The severity of acute COVID-19 in terms of symptoms and required treatment did not have an impact on the frequency of depressive symptoms in post-COVID-19 syndrome, however, it is important to note that age did no longer have a significant impact on melancholy rankings inside the examination through means of three studies. Notably, 13.0 weeks after the beginning of symptoms, patients with a milder form of the COVID-19 illness reported experiencing depressive symptoms more frequently than patients with a more severe form of the illness (22 percent vs. 10 percent).
A fourth study found similar findings, showing that nonintubated patients experienced more depressive symptoms than intubated patients (21.7 percent vs. 18.0 percent) at an average of 125 days following hospital release. However, no statistical analysis was ever carried out. Furthermore, there was a clear inverse relationship between the length of hospitalization and depression three months after discharge (ZSDS: r=-0.23, p=0.005, q=0.01; BDI-13: r=0.21, p=0.010, q=0.015), suggesting that shorter hospital stays were associated with worse depressive symptoms in the post-COVID-19 syndrome.
Depression and neurocognitive function
Depression is not a result of neurocognitive impairment during the acute phase of COVID-19. However, in the post- COVID-19 condition, depressive symptoms have a major impact on neurocognitive performance. A comparison between a group of patients with COVID-19 who experienced neurological problems while they were hospitalized and a control group of patients who did not. There were no discernible variations in depression scores between the two groups six to seven months following the beginning of neurological symptoms. In two different investigations, the association between depressive symptoms and neurocognitive function in the post-COVID-19 syndrome was investigated. It was shown that depressive patients often performed better on neurocognitive tests than those without symptoms. According to this study's token encoding test, wald=8.37, p=.003, greater ZSDS scores indicated slower processing speed and selective attention.
Discussion
Depressive symptoms and clinically severe depressive episodes in people with post-COVID-19 syndrome. At one month of followup, gender, psychiatric history, and psychopathology were all linked with the development and frequency of depression. Common risk factors for depression in the general population include the feminine gender, a history of depression, and other mental diseases. Findings regarding age as a moderator, however, varied.
At the 3 months follow-up following hospital release, depression symptoms were predicted by systemic inflammation at baseline, as determined by platelet count, levels of neutrophils, and lymphocytes (IBS), and its variations over time. According to recent research, the degree of inflammation in COVID-19 patients corresponds with the severity of symptoms during the acute phase. A greater prevalence of depressive symptoms in the post-COVID-19 syndrome was not shown to be related to the acute severity of COVID-19, as measured by the intensity of symptoms and the amount of care required, in this review COVID-19. Similarly, compared to hospitalizations without problems, COVID-19 patients who experienced neurologic difficulties during their stay did not have a statistically significant increase in the prevalence of long-term depression symptoms. Regarding the intensity of COVID-19 symptoms, it is uncertain if underlying systemic inflammation and depressive symptoms in the post-COVID-19 syndrome are related. Only a small portion of the post-COVID-19 depression hypothesis may be explained by systemic inflammation brought on by an acute infection. It has been shown that COVID-19 can result in a state that is very inflammatory and causes low-grade inflammation to persist.
More precisely, COVID-19 patients frequently have higher levels of IL-6 than controls do, as well as higher levels of TNF-, IFN-, IL-2, IL-4, IL-6, IL-10, and CRP. The most typical in COVID-19 patients, cytokines. Additionally, there is a link between mood disorder and depression, in particular, studies have shown higher levels of proinflammatory cytokines (such as Interleukin-6 (IL-6)) in symptomatic individuals.
Proinflammatory cytokines, according to the research currently available, decrease serotonin levels, Hypothalamic-Pituitary- Adrenal (HPA) axis autoregulation, glial microglial cells, and neuroplasticity in the nervous system, which results in a downregulation of brain function. Since cytokine storms are known to increase the risk of inflammation and significant consequences following SARS-CoV-2 infection in the elderly, this condition is frequently seen in this population. The effects of antidepressants and mindfulness practices on inflammation and depression in COVID-19 survivors require more study. To find potential therapy targets for depression in the post- COVID-19 condition, for instance, animal models might be created.
Furthermore, it is unclear if the high prevalence of depression among those with post-COVID-19 syndrome is an ongoing result of viral infection or an effect of the epidemic's social and/or economic effects. It has been established that the COVID-19 pandemic hurts indicators of general population mental health. For instance, since the commencement of the pandemic, greater incidences of depressed symptoms have been documented. Regional differences in the prevalence of depressed symptoms suggest that at the beginning of the pandemic when new public health restrictions were in place, it was probably significantly higher. A notable finding was that, according to data gathered during the first two weeks of the month, the prevalence of depressive symptoms in the United States during the COVID-19 pandemic was three times greater than it had previously been April 2020.
To compare the prevalence of depression in the post-COVID-19 syndrome with the prevalence of depression in the general community, longitudinal studies of COVID-19 patients in uninfected control groups are required. Only one of the trials that were included an infection-free control group, though. The date of the evaluation in this study did not coincide with the designation of post-COVID-19 syndrome in all patients, even though the study revealed increased depressive symptoms in COVID-19 patients (i.e., the time of assessment ranged from 12 to 215 days from diagnosis, with a mean of 126 days). Therefore, care should be used while interpreting the study's findings.
Despite this, it was noted that the prevalence of mood disorder was much greater six months after the diagnosis of COVID-19 than it was six months after the diagnosis of influenza or any respiratory illness. Its findings confirm the idea that SARS-CoV infection-2 increases the likelihood of long-term depression, even though this retrospective assessment of records was excluded from our results since it did not include data specifically for depression.
The onset and maintenance of depressive symptoms in patients with post-COVID-19 syndrome can be significantly influenced by social, economic, and geographic circumstances, to name a few. In addition to the virus's terror, the epidemic has brought social isolation, employment and housing insecurity, and school closures.
Due to the unusually severe social and economic effects of the pandemic, higher rates of depression have been seen in groups with lower socioeconomic status. Students reported making a similar remark, most likely as a result of the considerable transition from in-person training to online instruction. A lack of social networks, physical engagement in family and community activities, and isolation brought on by social isolation limits all have a detrimental impact on mental health. It should be mentioned that isolation policies are considered to contribute to younger people's (i.e., those under 40) greater incidence of depression. Psychosocial stresses that are already present cause inflammation. When considered together, the pandemic's economic and societal repercussions should be evaluated in the context of biological pressures.
RESEARCH IMPLICATIONS
There is not much information available at the moment on the prevalence of depression and the related post-COVID-19 syndrome. Studies are often required to evaluate the effects of post-COVID-19 syndrome on a bigger population, including control groups. A validated self-report questionnaire should be used in conjunction with a scale developed by a doctor to assess depression. The danger of overestimating and/or underestimating the actual incidence of clinically severe depression and depressed symptoms linked to post-COVID-19 syndrome will be reduced by using a scale that has been validated and certified by a physician. To track changes in symptoms over time, depression should also be evaluated upon diagnosis and at several points during recovery. Additionally, to enable adequate comparisons across studies, future research should strive to increase methodological consistency by sticking to a unified taxonomy of post-COVID symptoms.
Conclusions
This study's comprehensive evaluation showed that post- COVID-19 syndrome is frequently accompanied by clinically severe depression and depressive symptoms. Female gender, psychiatric history, and psychopathology at one-month follow-up were moderating variables. In the post-COVID-19 syndrome, the severity of COVID-19 and cognitive impairment during the acute illness are not linked to an aggravation of depressive symptoms. However, it cannot be said that people with post- COVID-19 syndrome experience depression more frequently than people in general. Therefore, the next research has to take post-COVID-19 syndrome categorization into account and incorporate a control group that hasn't been exposed to SARSCoV- 2. The overlap of depressive symptoms in patients with post-COVID-19 syndrome and their distinction from long-term COVID syndrome manifestations are also key study areas.
Limitations
There are several restrictions on this systematic review that might influence how our findings are interpreted. The included research was fairly diverse, to start. In patients from various nations and age ranges, depression was evaluated using various validated self-report questionnaires at various points after the diagnosis of COVID-19. Second, the majority of research lacked a control group (i.e., not exposed to SARS-CoV-2). Therefore, it is a testable hypothesis to determine if depression is a result of severe COVID-19 infection or a result of the social and/or economic effects of the pandemic. Multiple variables have also had an impact on depression during the epidemic (e.g., increased stress levels, financial difficulties, etc.). Third, neither the baseline (week 0) nor the period before acute infection was examined in these trials in terms of depressive symptoms of depression. The real prevalence of depression and clinically relevant depressive symptoms may be overestimated as a result of these limitations.
References
- Renaud-Charest O, Lui LM, Eskander S, Ceban F, Ho R, Di Vincenzo JD, et al. Onset and frequency of depression in post-COVID-19 syndrome: A systematic review. J Psychiatr Res. 2021;144:129-137.
[Crossref] [Google Scholar] [PubMed]
- Berk M, Williams LJ, Jacka FN, O'Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from?. BMC Med. 2013;11:200.
[Crossref] [Google Scholar] [PubMed]
- Chirumbolo A, Callea A, Urbini F. The effect of job insecurity and life uncertainty on everyday consumptions and broader life projects during COVID-19 pandemic. Int J Environ Res Public Health. 2021;18(10):5363.
[Crossref] [Google Scholar] [PubMed]
- Christensen KS, Sokolowski I, Olesen F. Case-finding and risk-group screening for depression in primary care. Scand J Prim Health Care. 2011;29(2):80-84.
[Crossref] [Google Scholar] [PubMed]
- Daher A, Cornelissen C, Hartmann NU, Balfanz P, Müller A, Bergs I, et al. Six months follow-up of patients with invasive mechanical ventilation due to COVID-19 related ARDS. Int J Environ Res Public Health. 2021;18(11):5861.
[Crossref] [Google Scholar] [PubMed]
- Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open. 2020;3(9):1-12.
[Crossref] [Google Scholar] [PubMed]
- Frontera JA, Yang D, Lewis A, Patel P, Medicherla C, Arena V, et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. 2021;426:1-8.
[Crossref] [Google Scholar] [PubMed]
- Gonzalez J, Benitez ID, Carmona P, Santisteve S, Monge A, Moncusi-Moix A, et al. Pulmonary function and radiologic features in survivors of critical COVID-19: A 3-month prospective cohort. Chest. 2021;160(1):187-198.
[Crossref] [Google Scholar] [PubMed]
- Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123-1130.
[Crossref] [Google Scholar] [PubMed]
- Ho CS, Chee CY, Ho RC. Mental health strategies to combat the psychological impact of Coronavirus Disease 2019 (COVID-19) beyond paranoia and panic. Ann Acad Med Singap. 2020;49(3):155-160.
[Google Scholar] [PubMed]
- Huang Y, Pinto MD, Borelli JL, Mehrabadi MA, Abrahim HL, Dutt N, et al. COVID symptoms, symptom clusters, and predictors for becoming a long-hauler looking for clarity in the haze of the pandemic. Clin Nurs Res. 2022;31(8):1390-1398.
[Crossref] [Google Scholar] [PubMed]
- Lee Y, Lui LM, Chen-Li D, Liao Y, Mansur RB, Brietzke E, et al. Government response moderates the mental health impact of COVID-19: A systematic review and meta-analysis of depression outcomes across countries. J Affect Disord. 2021;290:364-377.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Ho RC, Mak A. Interleukin (IL)-6, Tumour Necrosis Factor alpha (TNF-α) and soluble Interleukin-2 Receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J Affect Disord. 2012;139(3):230-239.
[Crossref] [Google Scholar] [PubMed]
- Liu D, Baumeister RF, Zhou Y. Mental health outcomes of coronavirus infection survivors: A rapid meta-analysis. J Psychiatr Res. 2021;137:542-553.
[Crossref] [Google Scholar] [PubMed]
- Loades ME, Chatburn E, Higson-Sweeney N, Reynolds S, Shafran R, Brigden A, et al. Rapid systematic review: The impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. J Am Acad Child Adolesc Psychiatry. 2020;59(11):1218-1239.
[Crossref] [Google Scholar] [PubMed]
- Lu Y, Ho CS, Liu X, Chua AN, Wang W, McIntyre RS, et al. Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS One. 2017;12(10):1-14.
[Crossref] [Google Scholar] [PubMed]
- Mattioli F, Stampatori C, Righetti F, Sala E, Tomasi C, de Palma G. Neurological and cognitive sequelae of COVID-19: A four month follow-up. J Neurol. 2021;268(12):4422-4428.
[Crossref] [Google Scholar] [PubMed]
- Mazza MG, de Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594-600.
[Crossref] [Google Scholar] [PubMed]
- Mazza MG, Palladini M, de Lorenzo R, Magnaghi C, Poletti S, Furlan R, et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun. 2021;94:138-147.
[Crossref] [Google Scholar] [PubMed]
- Morin L, Savale L, Pham T, Colle R, Figueiredo S, Harrois A, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325(15):1525-1534.
[Crossref] [Google Scholar] [PubMed]
Citation: Krishnamurthy M, Nagalakshmi NC, Raunakkumar C, Kumar Y (2023) Depression and Anxious during COVID-19. Health Care Curr Rev 12:396.
Copyright: © 2024 Krishnamurthy M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.

