Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 12, Issue 4
COVID-19 Vaccines: A Critical Appraisal
Saurav Deka, Betina Chandolia, Farah Iram, Niveditha Hariharan and Aparajita Dubey*Received: 17-Aug-2021 Published: 07-Sep-2021, DOI: 10.35248/2157-7560.21.12.460
Abstract
WHO declared the coronavirus outbreak as a global pandemic in March 2020, which has affected 220 countries across the globe and territories, with 169,597,415 confirmed cases and 3,530,582 deaths reported to date. SARSCoV- 2 vaccines are the best bet to control the pandemic and restore normalcy. Two of the mRNA vaccines got initial Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA), followed by the Advisory Committee on Immunization Practice (ACIP) initiative of publishing new guidance on vaccine priorities. Since millions of people are getting vaccinated, reports of temporary side effects and rare allergic reactions raised some troubling questions. Although there is no doubt related to the safety and efficacy of the current vaccines, the risk for the severe reaction gets entirely outweighed by the protection offered against the deadly coronavirus. This review article highlights upon the development of COVID-19 vaccines, their possible side effects, and management strategies.
Keywords
SARS-CoV-2; Detection; COVID-19 vaccines; Development; Mechanism; Side effects; Challenges; Management
Introduction
Since the establishment and coordination of national programs for immunization in the 1960s, vaccines have transformed public health [1]. Effective vaccination helped eradicate smallpox in humans and other diseases responsible for many childhood deaths. A vaccine is a biological product that can safely induce an immune response offering protection against any infection or disease on subsequent exposure to a pathogen. Most of the vaccines except BCG (which induces T cell responses) confer protection through the induction of antibodies. Generally classified as live or non-live, also referred to as inactivated to distinguish between the vaccines containing attenuated replicating strains of the relevant pathogenic organism to those containing components of a pathogen or killed organism. These several other platforms, including viral vectors, nucleic acidbased RNA and DNA vaccines, and virus-like particles, have been developed over the past few decades. Immune memory is a crucial feature of vaccine-induced protection. Typically, post-primary vaccination, the antibody levels in the circulation wane out often to a level below that required for safety. Hence, immune memory depends on the incubation time of the infection, the quality of the memory response, and the level of antibodies induced by memory B cells.
Herd immunity
Herd immunity is another essential feature of vaccine-induced protection. Applicable for highly contagious diseases such as measles and other infectious diseases such as COVID-19 at present. It includes susceptible individuals who have not been yet immunized (for example, younger age group), immunocompromised, for whom the vaccine did not induce immunity, and those who refused immunization.
Several vaccines such as MMR (Measles Mumps Rubella), Polio, Typhoid, BCG, Tetanus (DTP), Yellow fever, Smallpox, Rabies, Flu, Hepatitis A, B, C, Cervical Cancer, Human Papilloma Virus (HPV), Shingles, Chicken Pox, Pneumonia, HSV, etc. developed in the past [2]. The antibody production induces the immune response against the antigens present in the pathogens. Protective levels determined through controlled clinical trials with a defined International standard.
Lessons learned from previous viral vaccines
Viruses adapt to immune pressures very efficiently. Most viruses utilize multi-step approaches to infect cells involving multiple viral proteins and multiple receptors on cells. High immune pressure is responsible for frequent mutations. Induction of neutralizing antibodies requires the activation of several components of the immune system. Protective immunity cannot be achieved only by neutralizing antibodies. Several factors are involved in vaccineinduced responses, including vaccine product-related components or host health and immunological/genetic condition. Neutralizing antibodies need to be directed to both binding and fusion domains of the virus. It has been highly challenging to make an antibody to the "fusion" domain of the influenza virus, and hence, we do not have a "universal influenza vaccine" to date. In the current pandemic caused by the virus SARS-CoV-2, vaccines preventing severe disease and disease-driven hospitalization are having a substantial public health impact. However, a vaccine that could also restrict the acquisition of the virus, preventing both asymptomatic and mild infection, is having a significant impact by reducing transmission in the community and potentially establishing herd immunity.
Critical questions in designing the suitable vaccine?
To design the right vaccine, we should be sure about the nature of the protective immune response, whether it should be humoral (antibodies) or cell-mediated (cytotoxic T cells)? What part of the virus can induce this immune response? What kind of vaccine, dose, frequency, and quantity is required?
Criteria for an “Ideal-vaccine”
• It should stimulate antibody responses of neutralizing antibodies in the titer range of ~2560 for greater than one year.
• It should stimulate a memory CD4 and CD8 T and B cells response.
• It should have a significant effect size in a population that can enable herd immunity, e.g., >70%.
• It should induce protection for children, older adults, and patients with comorbidities, all of whom may have sub-optimal immune responses.
• It should be safe and not results in acute hypersensitivity reactions, e.g., in <0.1%.
• It should have a formulation that makes the vaccine stable in extreme temperature and distribution conditions.
• It should induce mucosal immunity in the oral-respiratory tract [3].
SARS-CoV-2 virus
The SARS-CoV-2 virus consists of four structural proteins: Spike (S), an Envelope (E), Hemagglutinin Esterase (HE), Membrane (M), and a Nucleocapsid (N) Protein. SARS-CoV-2 virus entry, viral replication machinery, viral lifecycle, translation of viral structure protein, virion assembly, and release mainly describes the life cycle of the SARS-CoV-2 virus (Figure 1) [4].
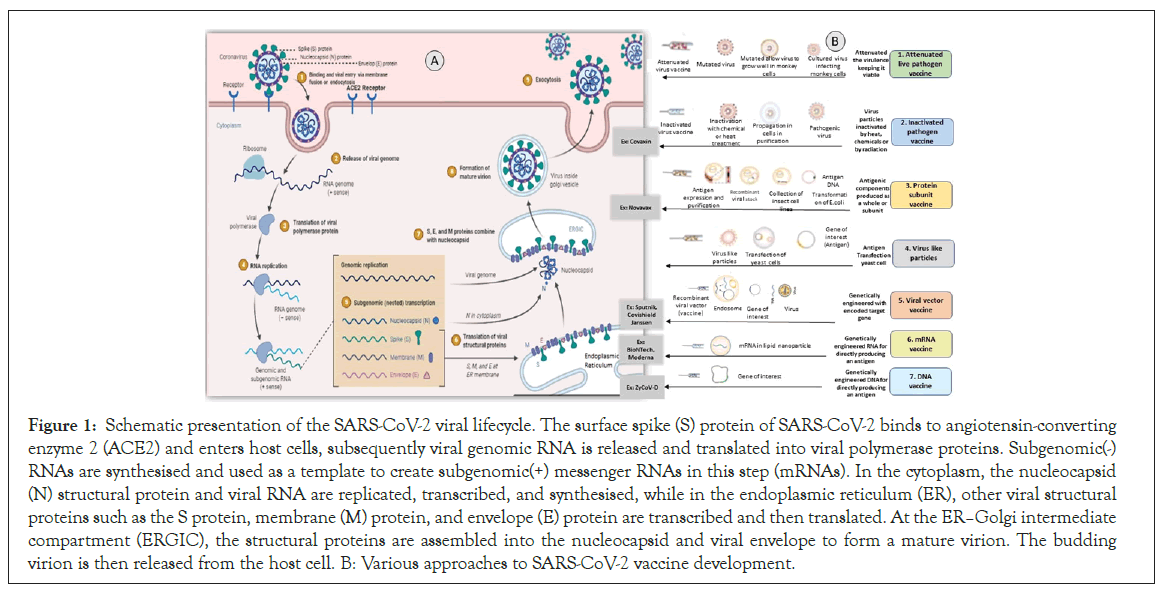
Figure 1: Schematic presentation of the SARS-CoV-2 viral lifecycle. The surface spike (S) protein of SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2) and enters host cells, subsequently viral genomic RNA is released and translated into viral polymerase proteins. Subgenomic(-) RNAs are synthesised and used as a template to create subgenomic(+) messenger RNAs in this step (mRNAs). In the cytoplasm, the nucleocapsid (N) structural protein and viral RNA are replicated, transcribed, and synthesised, while in the endoplasmic reticulum (ER), other viral structural proteins such as the S protein, membrane (M) protein, and envelope (E) protein are transcribed and then translated. At the ERâ??Golgi intermediate compartment (ERGIC), the structural proteins are assembled into the nucleocapsid and viral envelope to form a mature virion. The budding virion is then released from the host cell. B: Various approaches to SARS-CoV-2 vaccine development.
Detection and stage of disease
Three categories of testing exist at present. The first one is to identify whether the actual COVID-19 virus genetic material exists, called a NAAT test, N-A-A-T. It is the PCR testing where a nasal pharyngeal swab or a pharyngeal swab is collected. Then to look for the genetic material of the virus second type is called antigen testing, to identify one of the outer proteins of the viral shell or envelope. And the third type is to detect whether the human body has developed antibodies. It looks for antibodies specific to the outer portion of the virus itself to show whether the individual has mounted an immune response or acquired immunity towards that virus or to COVID-19.
There are three stages of disease progression: Asymptomatic, moderate, and severe. In asymptomatic, the viral load is low, whereas moderate can have fever due to cytokine storm (IFNα, IL6 test) along with blood clotting (Ferritin, D-Dimer test) O2- Saturation. Anti-viral and anti-inflammatory drugs can be helpful during the asymptomatic and moderate stages of the disease. The third or severe stage of disease results in "lymphopenia" and all other symptoms (T cell responses, NAB responses) and finally leads to multi-organ failure [5].
Type of COVID-19 vaccines in development
Significant steps involved in any vaccine development involve antigen identification, expression, process development, animal toxicology study, clinical trials, regulatory review, approval, followed by scale-up and distribution. The global vaccine pipeline has more than 100 candidates being developed against SARSCoV- 2 by research teams in companies and universities worldwide. Researchers are using different technologies, some of which have not been used in a licensed vaccine before. The primary aim of all vaccines is to expose the body to an antigen that won't cause disease but will elicit an immune response against the virus. The significant type of vaccines under development against the coronavirus relies mainly on different viruses or viral parts [6].
Mechanism of Action
In an immunized individual humoral and cellular immune response is induced by vaccination. Usually, the homologous virus gets neutralized or cleared when it enters an immunized body respectively by vaccine-induced neutralizing Antibodies (Abs) or specific T cells [7,8].
When homotypic or heterotypic serotype viruses challenge these vaccinated individuals, the antibodies will immediately recognize the viruses and mediate antibody-dependent disease aggravation. There are various approaches to SARS-CoV-2 vaccine development (Figure 1) [9].
Live attenuated vaccine
These vaccines contain viruses modified to hamper their replication and infection rate. Such vaccines create mild viral infections with the consequent anti-viral response. It induces a robust immune memory response without any reminders. It Induces an innate immune response and produce proinflammatory cytokines (IL-1β, TNF, and IL-6) [7,8].
Inactivated vaccine
Inactivated vaccines constituted by a virus treated physio chemically to inhibit its pathogenicity. Upon injection, the inactivated viruses are engulfed by Antigen Processing Cells (APCs), and different epitopes are presented to the immune system. It induces T-cell responses and cytokine production such as IFN-γ, TNF-α, IL-5, IL- 4, IL-2 [10].
Protein subunit vaccine
The subunit vaccines are composed of viral surface proteins formulated with adjuvants to elicit strong neutralizing antibody responses. Once injected, the proteins are engulfed by the APCs [7,9].
Virus-like particles (vlp) vaccine
They are constituted by one or more viral proteins assembled into a particle without viral genetic material. These viral particles have a better uptake profile and more efficient circulation to the lymph nodes cause disease. Thus, they produce stronger immune responses. It induces neutralizing antibody responses, Th1 and Th2 cell immediate immunity [11].
Viral vector vaccine
Recombinant virus vaccines composed of viral vectors containing proteins from the target virus. It completely mimics natural viral infection, creating a strong immune response against it. It induces anti-spike IgG responses against it. It induces anti-spike IgG responses and spike-specific T-cell responses and activates cytotoxic T cells [11].
mRNA vaccine
These vaccines deliver RNA coding targeting viral proteins into human cells. Where they produce viral protein and, if encoded by polymerase, replicate themselves. This way, RNA-based vaccines mimic ongoing viral infection, including toll-like receptor activation and IFN production. Hence, vaccines with different mRNA versions of the spike protein have been developed. It induces CD4 T cell responses, neutralizing antibodies, induces the robust CD8+ T cell and Th1 [8,10].
DNA vaccine
DNA vaccines allow for the rapid design of multiple candidates for novel antigens. Either they are directly injected or inoculated into recipients, making them appropriate for emerging infectious diseases. It induces T cell activation by delivering DNA plasmids that express the SARS CoV-2 S protein. The effects are based on measuring functional antibodies and antigen-specific T cell response. Upon expression of the transgene, APCs present antigenic peptides that initiate humoral and cellular immunity. Plasmid DNA vaccines expressing SARS-CoV-2 S protein generated neutralizing antibodies and antigen-specific CD8+ T cells [10,11].
COVID-19 Vaccine and Immunogenicity
The virus enters through the nasopharyngeal route into the lungs and enters cells through the ACE2 receptor. Cytopathic viral replication takes place inside the cell and induces immune inflammation resulting in cytokine secretion (IL1, IL6, TNF α) storm. COVID-19 vaccines safely produce an immunogen (an antigen capable of eliciting an immune response) to the immune system, training it to identify the pathogen when it is confronted naturally by triggering: CD4+ helper T cells that in turn stimulate B-cells to generate neutralizing antibodies specific to the virus and CD8+ cytotoxic T cells to identify and kill cells affected by the virus. The cascade of immune responses induced by various COVID- 19 vaccines can be understood in Figure 2 [12-14].
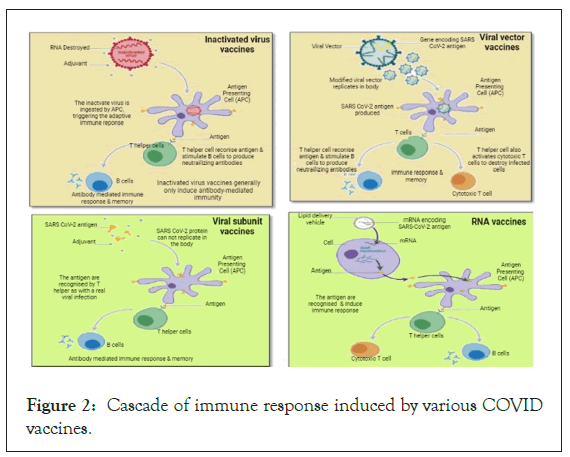
Figure 2: Cascade of immune response induced by various COVID vaccines.
List of approved/authorized vaccines
With the advent of high-end and path-breaking technologies, the world could see COVID-19 vaccines in a short period. A vaccine is efficacious if it prevents further morbidity and mortality in the country [14]. Nearly 166 vaccines candidate is in preclinical development [15], and Table 1 [15-17] lists the vaccines that are already approved or authorized. Pre-approval in the market, the efficacy and safety of the vaccine have been vigorously tested in multiple groups (Table 2) [18-37]. There are many more vaccines in phase 3 of development, a few of which are listed in Table 3 [38].
| Vaccine type (Platform) | Vaccine | Manufacturers | Country of origin | Antigen type | Storage conditions | Vaccine administration and dose | Advantages of the vaccine type | Disadvantages of the vaccine type | Cost |
|---|---|---|---|---|---|---|---|---|---|
| 1. mRNA | 1. mRNA-1273 2. BNT162b2 |
Moderna US Pfizer-BioNTech (US) | US Multinational | Full-length spike (S) protein with proline substitutions Full-length S protein with proline substitutions | -25° to -15 °C; 2-8 °C for 30 days; room temperature ≤ 12 hours -80° to -60 °C; 2-8 °C for 5 days; room temperature ≤ 2 hours | Two doses, 28 days apart Dose: 100 µg Intramuscular injection, two doses, 21 days apart Dose: 30 µg | Easy standardization and scaling up Safety and simple manufacturing | Storage and transportation issues | 25-37USD per dose 19.50 USD per dose |
| 2. Replication defective viral vector vaccine | 1. ChAdOx1
(AZS1222) 2. Gam-COVID-Vac (Sputnik V) 3. Ad26.CoV2.S 4.Convidicea (Ad5-nCoV) |
AstraZeneca / Oxford Gamaleya National Research Center for Epidemiology and Microbiology Janssen/ Johnson and Johnson CanSino Biologics | UK Russia The Netherlands, United States China | Replication-deficient chimpanzee adenoviral vector with the SARS-CoV-2 S protein Full-length SARS-CoV-2 glycoprotein S carried by adenoviral vectors. Recombinant, replication incompetent human adenovirus serotype 26 vector encoding a full-length, stabilized SARS-CoV-2 S protein Recombinant vaccine (adenovirus type 5 vector) | 2-8 °C for 6 months -18 °C (Liquid form); 2-8 °C (freeze dried) for up to 6 months -20 °C; 2-8 °C for 3 months 2-8 °C | Two doses, 4-12 weeks apart Dose: 0.22 ml or 0.5 ml Two doses, 21 days apart Dose: 0.5 ml or 1.0 ml 1 dose, Dose:1 ml Single dose vaccine | Viruses are modified to reduce their virulence Provides better immune response | Complex to manufacture | $2.15 (U.S.) in the EU; $3-4 (U.S.) in the UK and U.S.; $5.25 (U.S.) in South Africa. 10 USD per dose 10 USD per dose 27.15 USD per injection |
| 3. Inactivated pathogen vaccine | 1. CoronaVac, 2. BBIBP- CorV/Sinopharm 3. Covaxin |
Sinovac Biotech Beijing BioInstitute of Biological Products Bharat Biotech, ICMR, Ocugen | China China India | Inactivated CN02 strain of SARS-CoV-2 created from Vero cells Beta-Propiolactone inactivated. Inactivated virus mixed with aluminium based adjuvant. 6 µg of whole-virion. Inactivated SARSCoV-2 antigen (Strain: NIV-2020-770), and the other inactive ingredients such as aluminum hydroxide gel (250 µg), TLR 7/8 agonist (imidazoquinolinone) 15 µg, 2-phenoxyethanol 2.5 mg, and phosphate ® buffer saline up to 0.5 ml. | 2-8 °C; Lifespan Unknown 2-8 °C; Lifespan Unknown 2-8 °C | Two doses, 14-28 days apart 3 μg With Aluminium hydroxide adjuvant 2 Doses 21 days apart 4 μg with aluminum hydroxide adjuvant Two dose vaccination, 28 days apart Dose- Not available | A dead form of the pathogen is used and hence a better safety profile is ensured. | Chemically inactivated pathogen may lose immunogenicity and addition of adjuvants is necessary. | 60USD 145 USD for two doses 400 to 1200 INR |
| 4. Protein subunit vaccine | NVX-CoV2373 | Novavax, Inc | United States | Recombinant full-length, prefusion S protein | 2-8 °C for 6 months | 5 μg of protein and 50 μg of Matrix-M Adjuvant | Elicits better immunogenicity Eliminates the possibility of severe side effects | Booster doses are required | $16 in the US (17) |
Table 1: List of Approved/Authorized COVID-19 vaccines.
| Name of the Vaccine | Details of Phase trials | Subject Age | Efficacy of the Vaccine | Comments |
|---|---|---|---|---|
| 1. mRNA-1273/Moderna US | 1. Phase 1 Trial (NCT04283461)- 105 healthy participants 2. Phase 2 (NCT04405076) - 600 healthy participants. 3. Phase 3 (NCT 004470427)- 30,000 participants at high risk of COVID-19 |
18 years or older (including >55 years) | In a phase 3 trial ((NCT 004470427) involving 30,420 volunteers (15,210 participants in vaccine and placebo group), it was identified that m-RNA 1273 vaccine demonstrated 94.1% efficacy at preventing the COVID-19 illness (18). A recent study identified that moderna vaccine can also neutralize the Indian variant B.1.617.1 (19). | The vaccine is found to be effective in patients with a history of COVID-19 infection. The efficacy of Moderna vaccine is yet to be validated in more longitudinal follow-up studies involving pregnant woman. |
| 2. BNT162b2/Pfizer BioNTech US | 1. Phase 1 and 2 trials (NCT04380701) in US and Germany- 200 healthy participants 2. Combined phase 1 and 2 trials (NCT04588480) - 160 participants in Japan 2. Phase 2 trial 960 participants in China (NCT 04649021) 3.Phase 2/3 (NCT04368728) trial 43,548 healthy volunteers |
16 years or older (including >55 years) | In a study involving 43,548 participants (21,720 with BNT162b2 and 21,728 with placebo), it was found that BNT162b2 was 95% effective in preventing COVID-19. This vaccine was not only effective in reducing hospitalization among healthcare workers in UK (20) but was also found to be effective in preventing hospitalization among patients who are older than 65 years of age in the United States (21). Researchers identified that this vaccine was found to be 89.5 % effective against the B.1.1.7 variant and 75% effective against B.1.351 variant (22). | The vaccine is been tested in older adults and pregnant women and it shows promising results. |
| 3. COVID-19 Vaccine AstraZeneca (AZD 122) (Vaxzevria in Europe and CoviShield in India) | 1. Phase 1 trial (NCT 04324606)- 1090 healthy adult volunteers 2. Phase 2/3 trial (NCT 04400838)- 12,390 participants 3. Phase 3 trial (NCT04516746 ) – 32,000 participants |
18 years or older (including >55 years) | An interim analysis of four randomized controlled trials in Brazil, South Africa, and UK showed that participant who received two standard doses had a vaccine efficacy of 62.1%. (23). Also, in a prospective cohort study (24) conducted by Vasileiou et al., it was observed that ChAdOx1 vaccines are associated with substantial reductions in the risk of hospital admission due to COVID-19 in Scotland. Similar to other SARS-Cov2 lineages, this vaccine is found to be effective against the B.1.1.7 variant of SARS-CoV-2 (25). However, a study conducted by Madhi et al., identified that a two-dose regimen of ChAdOx1 nCoV-19 did not offer protection against mild-to-moderate COVID-19 due to the B.1.351 variant that originated in South Africa (26). | The efficacy of this vaccine in pregnant and lactating women is yet to be established. It is important to determine which subset of patients develops thrombotic events as side effects to this vaccine. |
| 4. Sputnik V | 1. Phase 1 and 2 trials (NCT 04436471 and NCT 04437875)- 38 participants, 2. Phase 3- 21,977 participants. |
18-60 years | In a randomized, double-blind, placebo-controlled, phase 3 trial on 21,977 participants it was identified that Sputnik V showed 91.6% efficacy (27). | Participants in the phase 1 and 2 clinical trials appear to be low compared with the rest of the vaccine trials on COVID-19. The efficacy of this vaccine in patients with comorbid conditions, pregnant and lactating women is still to be established. The efficacy of this vaccine in participants elder than 60 years is been evaluated in a phase 2 trial (NCT04530396) of 110 participants. |
| 5. COVID-19 Vaccine Janssen (JNJ-78436735;Ad26.COV2.S) | Phase 3 ENSEMBLE trail – 43,783 participants | 18 years or older (including >55 years) | In a phase 3 randomized, double-blind, placebo-controlled trial involving 19,630 SARS-CoV-2–negative participants it was identified that a single dose of Ad26.COV2.S vaccine was effective against symptomatic COVID-19 and asymptomatic SARS-CoV-2 infection. This vaccine was also found to be effective against severe disease (28). | During April 2021, 6 out of 6.8 million vaccinated individuals developed cerebral venous sinus thrombosis and thrombocytopenia. |
| 6. Convidicea (Ad5-nCoV) | 1. Phase 1 trial- China-108 participants (NCT 04313127) 2. Phase 2 trial- 508 participants (NCT 04341389) 3. Phase 3 trial- 40,000 participants (NCT04526990) 4. Phase 4 trial-120 participants (NCT 04833101) |
18-55 years |
In an interim analysis of phase 3 trial it was identified that this vaccine was 65.7% effective in preventing symptomatic cases (29). | The efficacy of the vaccine need to be evaluated in pregnant and lactating women, and in patients with comorbidities. |
| 7. CoronaVac | 1. Phase 1 trial (NCT04352608)- 143 participants 2. Phase 1/2 trial (NCT04383574)- 600 healthy volunteers 3. Phase 3- 9000 patients (NCT04456595 in Brazil, NCT 04582344 in Turkey, and NCT04508075 in Indonesia). |
18-59 years | The efficacy of this vaccine varies according to the region in which the phase 3 clinical trials were conducted. The efficacy ranged between 50% and 83.5%. The phase 3 trials conducted in Brazil and Turkey indicated that the vaccine prevented 100% hospitalizations and was 50.65% effective in preventing infection. CoronaVac was also found to be effective against the B.1.1.7 variant, but was less effective against the South African variant B.1.351 (30, 31). | CoronoVac was found to be safe and well tolerated in older adults (30) |
| 8. BBIBP- CorV | 1. Phase 1 – 192 adults 2. Phase 2- 448 adults ChiCTR2000032459 3. Phase 3 ChiCTR2000034780 (China) NCT04560881 (Argentina)Trial with 31,000 volunteers in the UAE in collaboration with G42 Healthcare |
18-59 years | The efficacy of the vaccine was found to 79% against symptomatic SARS-CoV-2 infection (32). Wang et al., (33) reported that this vaccine was found to be effective against the B.1.1.7 variant originating in the UK. |
The efficacy of this vaccine in patients with comorbidities and in elder patients is yet to be determined. |
| 9. Covaxin (BBV152) | 1. Phase 1,2 trial (NCT 04471519) – 375 in trail 1 and 380 in phase 2 healthy participant 2. Phase 3 trials – 26,000 participants |
18 years or older (including >60 years, >4500 people with comorbid conditions | The results of phase 3 trials on covaxin identified that this vaccine is 78% effective in preventing COVID-19 in people without any history of COVID infection (34). The vaccine is also safe against the B.1.1.7 variant of SARS-Cov-2 (35). | The effect of covaxin has not been studied in pregnant woman and nursing mothers. |
| 10. Novovax | 1. Phase 1 trial (NCT04368988) – 130 healthy participants 2. Phase 2b – 2665 adults (NCT 04533399) 3. Phase 3 – PREVENT trial (NCT04611802) |
18 to 59 years | Results from phase 2b clinical trial showed that the efficacy of the vaccine was 60% in HIV negative participants (36). | The phase 2 trial includes 240 adults who are HIV positive. The phase 3 trial is being conducted in United States and Mexico and includes pediatric patients aged 12 to 17 years |
Table 2: Efficacy of the approved or authorized vaccines.
| Name of the Vaccine | Vaccine type | Sponsor | Institution | Details of clinical trails |
|---|---|---|---|---|
| Sputnik Light | Recombinant Adenovirus vaccine (rAd26) | Gamaleya Research Institute, Acellena Contract Drug Research and Development | Russia | Phase 3 (NCT 04741061)- 6000 participants |
| ARCoV | mRNA based vaccine | Walvax Biotechnology Co., Ltd.; Abogen Biosciences Co. Ltd. | Xiangfen CDC | Phase 3 |
| NVX-CoV2373 | Nanoparticle vaccine | Novavax | Novavax | Phase 3 |
| ZyCOV-D | DNA vaccine (plasmid) | Zydus Cadila | Zydus Cadila | Phase 3 |
| bdala (CIGB66) | Protein subunit vaccine | Center for Genetic Engineering and Biotechnology | Center for Genetic Engineering and Biotechnology | Phase 3 |
| Unnamed | Plant-based adjuvant vaccine | Medicago; GSK; Dynavax | Medicago | Phase 3 |
| VLA2001 | Inactivated vaccine | Valnela; UK National Institute for Health Research | Multiple NIHR testing sites | Phase 3 |
| No name announced | Adjuvant protein subunit vaccine | Biological E, Baylor College of Medicine, Dynavax, CEPI | Various | Phase 3 |
| No name announced | Inactivated vaccine | Chinese Academy of Medical Sciences, Institute of Medical Biology | West China Second University Hospital, Yunnan Centre for Disease control and prevention | Phase 3 |
Table 3: Vaccine candidates in Phase 3 and Phase 4 of development: Some of the potential vaccines that are in phase 3 and 4 development are listed below.
Post-vaccination challenges
The trials of the vaccines are being carried out for safety and efficacy in the individuals suffering from COVID-19 the aim of developing vaccines is to protect COVID-19. However, these vaccines' benefits come with challenges, including getting infected with COVID-19 even after getting vaccinated, side effects, and adverse effects.
Individuals are getting infected with COVID-19 even after COVID-19 vaccination. One of the reasons for this could be the body's time to develop protection against the virus, which is two weeks. Another reason for getting COVID-19 after vaccination is the attack of a new variant of the COVID-19 virus on the body for which the COVID-19 vaccination might not be effective. However, if a person gets COVID-19 after vaccination, the chances of getting severely sick are lesser than the person who is not vaccinated with COVID-19 vaccine [39].
Till May 18, 23,940 people in India got the infection after taking both shots of Covaxin, which is 0.13 percent of the total vaccinated so far. After the first dose of Covaxin, 18,427 tested positive and 5,513 after the second dose. In the case of persons receiving Covishield, the number of people infected after vaccination was 1,19,172, which was 0.07 percent of the total. After the first dose of Covishield, 84,198 people tested positive, 34,874 were infected after their second shot. As per the availability of data till May 18, 1,90,90,274 (1.9 crores) doses of Covaxin were administered, and 16,47,64,399 doses of Covishield were given in India [40].
Side-effects of COVID-19 vaccine
According to Riad et al. [41] the side-effects of the COVID-19 vaccine can be local or systemic, or both. The severity of side effects can vary from mild to moderate. In very rare cases, these side effects can become severe.
General side effects
The side effects experienced due to each COVID-19 vaccine can vary. As per Riad et al., the side effects of the COVID-19 vaccine include pain, redness and swelling at the injection site, fatigue, headache, muscle pain, chills, fever, nausea, and feeling of sickness. Other side effects include enlargement of lymph nodes and oral side effects [42].
Oral side-effects
The oral side-effects included blisters, halitosis, ulcers, angular cheilitis, bleeding gingiva, tongue-tingling, taste disturbances, vesicles, xerostomia, presence of white or red plaque, and swollen lips.
Lips are the most common location for the occurrence of blisters, vesicles, and ulcers. Other sites included palate, labial/buccal mucosa, tongue, and gingiva. The location for white/red plaque occurrence included dorsum of the tongue, soft palate, and labial/ buccal mucosa [43].
Skin side-effects
The most common side-effects on skin included skin rashes and urticaria. The location of these side-effects included upper limbs, lower limbs, chest/trunk, face, and back region [44].
The side-effects of Sputnik V, Moderna, Pfizer-BioNTech, Covaxin, and Covishield [45].
Sputnik V:
• Headache
• Fatigue
• Pain at Injection Site
•Flu-like illness
Moderna:
• Fever
• Chills
• Redness at Injection Site
• Swelling at Infection Site
Pfizer-BioNTech:
• Headache
• Myalgia
• Arthralgia
• Injection Site Pain
• Fatigue
• Chills
• Fever
Covaxin:
• Redness, Swelling, Pain at the Injection Site
• Fever
• Sweating or Chills,
• Headache
• Malaise, Body ache
• Nausea and Vomiting
• Itching and Rashes
Covishield:
• Pain at the Injection Site
• Redness
• Moderate or high fever
• Drowsiness and Lethargy
• Arm stiffness
• Body ache and pain
The comparison of side effects after vaccination from Covishield and Pfizer BioNTech is shown in Figure 3. The comparison of sideeffects of Johnson and Johnson, Pfizer BioNTech, and Moderna Vaccine after second dose is shown in Figure 4.
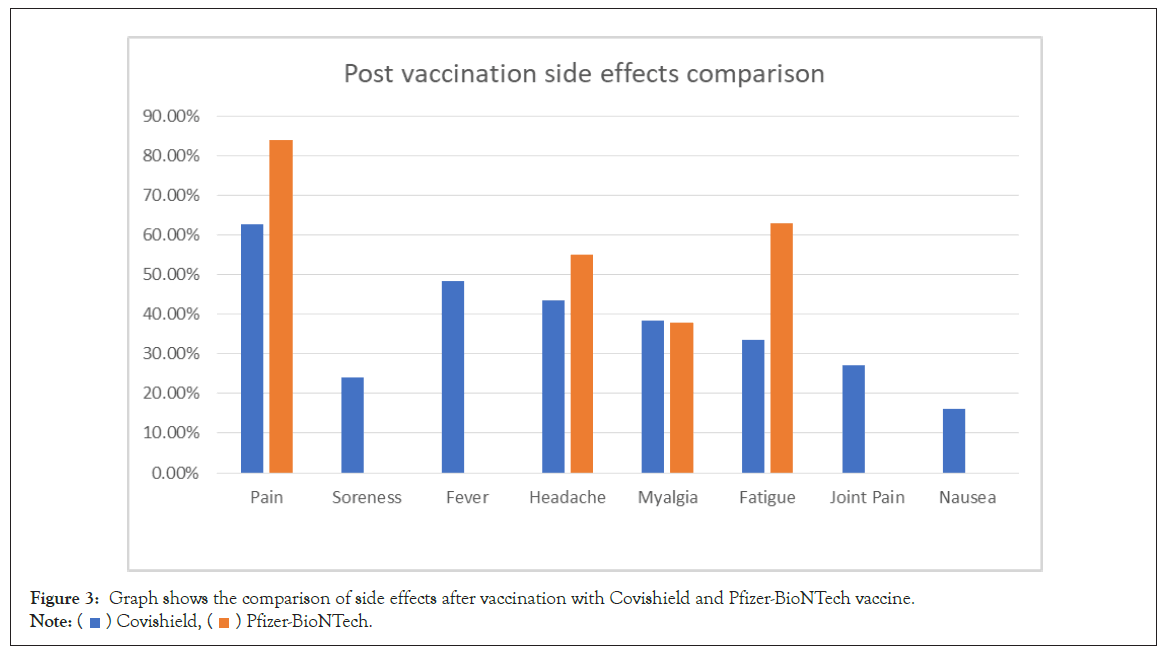
Figure 3: Graph shows the comparison of side effects after vaccination with Covishield and Pfizer-BioNTech vaccine.
Note:  Covishield,
Covishield,  Pfizer-BioNTech.
Pfizer-BioNTech.
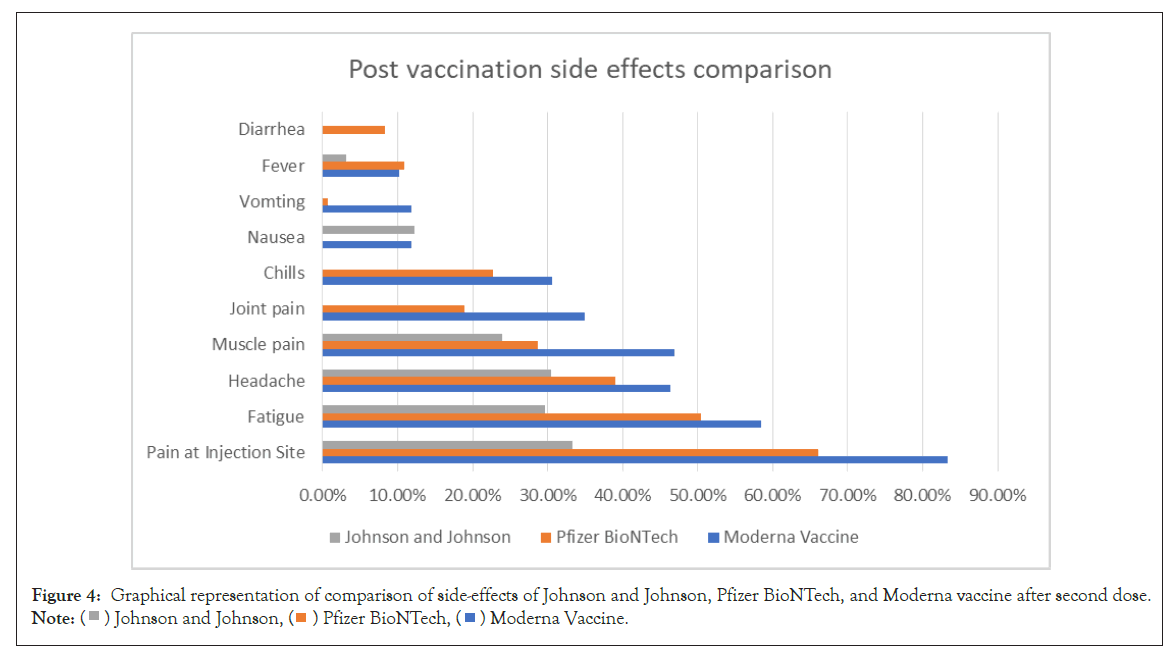
Figure 4: Graphical representation of comparison of side-effects of Johnson and Johnson, Pfizer BioNTech, and Moderna vaccine after second dose.
Note:  Johnson and Johnson,
Johnson and Johnson,  Pfizer BioNTech,
Pfizer BioNTech,  Moderna Vaccine.
Moderna Vaccine.
Rare/Severe Side-Effects
The severe side effects of vaccines include severe headache, which is not helped by painkillers, worsening of headache on lying or bending down, unusual headache accompanied by blurred vision/ nausea and vomiting/difficulty with speech/weakness/drowsiness/ seizures/bruising/bleeding/shortness of breath/chest pain/leg swelling/persistent abdominal pain. Rare side effects include acute pancreatitis and immune thrombotic thrombocytopenia.
Adverse effects of COVID-19 vaccines
Adverse effects of COVID-19 vaccines are potentially lifethreatening consequences of vaccination. The reported orofacial adverse effects include swelling of lips, face, tongue, or throat. The rare adverse effect reported is Bell's palsy. In the case of people who had facial cosmetic injections, swelling of the face be an adverse effect post COVID-19 vaccination [46].
Controversy regarding antibody-dependent enhancement
The Antibody-Dependent Enhancement (ADE) of disease after vaccination is insufficient to predict the adverse outcomes in humans. The protective effect of antibodies depends on two factors: binding of viral proteins by their Fab (fragment, antigen-binding) fragments and on the effector, functions conferred by their Fc (fragment, crystallisable) fragments. In vaccine formulations that have shown disease enhancement, such as formalin inactivation, the neutralizing antibodies with optimized properties are protective. However, the ADE potential mechanisms are virus-specific; the clinical markers cannot differentiate severe infection from immune enhancement. No cases of ADE have been reported in the patients of COVID-19 vaccine to date during the preclinical animal studies and human clinical trials. No signs have been observed during the initial vaccine rollouts into the population. So far, no sign of ADE has been noted, even with the variant strains in different parts of the world. So far, there is no clear evidence that ADE may be involved in the immunopathological processes associated with COVID-19 or re-infection with other variants after recovery or post-vaccination. More studies are required to understand the correlation of protection against SARS-CoV-2 in natural human infection and as vaccines and antibodies in humans [47].
Management of side-effects/adverse effects
After getting vaccinated for COVID-19, the management of sideeffects is important to avoid any kind of serious adverse health effects.
The most common side-effect after getting the COVID-19 vaccine is pain and discomfort at the injection site. It is advised to apply a clean, cool, wet washcloth over the area to reduce it. The vaccinated arm should be used and exercised as per the advice of the doctor.
Fever is another common side-effect after getting the COVID-19 vaccine. It is essential to drink plenty of fluids to avoid the side effects. To alleviate the side effects such as pain, tenderness, fever, and fatigue, paracetamol is advised. In case of serious side-effects or adverse effects, it is advised to seek medical advice immediately [48].
Discussion and Conclusion
With nearly a dozen COVID-19 vaccines already approved or authorized, around 166 vaccines are in the preclinical development phase. It's challenging to make head-to-head comparisons to rate the vaccines as the technology used and the ethnic groups on which the trials were done are different. mRNA vaccines show greater efficacy, but their limitation lies in storage and transportation. Replication defective viral vector vaccines have demonstrated a good efficacy against COVID-19 infections, but they are a bit difficult to manufacture. Inactivated pathogen vaccines use traditional techniques and have shown promising results. There are many more protein subunit vaccines to reach the market soon.
Traditionally, vaccine development takes more than a decade, but the COVID-19 pandemic has demonstrated enormous urgency for flexible vaccine technologies that facilitate rapid development, production, and scale-up.
The focus is on viral vectored vaccines, nucleic acid-based vaccines, antigen-presenting cells such as dendritic cells, T cell-based vaccines, and bacterial vectors. These vaccines generate an immunogen to the immune system, training it to identify the pathogen when encountered naturally by prompting CD4+ helper T cells that stimulate B-cells to induce neutralizing antibodies specific to the virus and CD8+ cytotoxic T cells to recognize and eradicate cells influenced by the virus.
The trials have proven the efficacy of the COVID-19 vaccines; however, post-vaccination challenges such as side/adverse effects cannot be ignored. To date, no case of ADE has been reported in trials. However, more studies are required to understand the correlation of protection against SARS-CoV-2, to evaluate the efficacy of COVID-19 vaccines in children, pregnant women, and lactating mothers to reach a better conclusion.
REFERENCES
- Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments.Nat Rev Immunol.2021;21(2):83-100.
- Comparative analysis of emerging viruses to inform development of safe and effective vaccines for COVID-19.2021.
- Soleimanpour S, Yaghoubi A. COVID-19 vaccine: Where are we now and where should we go? Expert Rev Vaccines. 2021;20(1):23-44.
- Le TT, Andreadakis Z, Kumar A, Román RG, Tollefsen S, Saville M, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305-306.
- Grigoryan L, Pulendran B. The immunology of SARS-CoV-2 infections and vaccines. Semin Immunol 2020.50: 101422.
- Callaway E. The race for coronavirus vaccines: A graphical guide. Nature.2020;580(7805):576-577.
- Su S, Du L, Jiang S. Learning from the past: Development of safe and effective COVID-19 vaccines. Nat Rev Microbiol.2021;19(3):211-219.
- Rodriguez-Coira J, Sokolowska M. SARS-CoV-2 candidate vaccines - composition, mechanisms of action and stages of clinical development. Allergy.2020.
- Awadasseid A, Wu Y, Tanaka Y, Zhang W. Current advances in the development of SARS-CoV-2 vaccines. Int J Biol Sci. 2021;17(1):8-19.
- Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6(1):28
- Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2020.170:1-25
- Update 45-vaccines-developement.pdf.2021.
- Teijaro JR, Farber DL. COVID-19 vaccines: Modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195-197.
- Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21(2):e26-e35.
- Coronavirus disease (COVID-19)-World Health Organization.2021.
- UPDATED Comparing COVID-19 Vaccines: Timelines, Types and Prices .BioSpace. 2021.
- Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 Vaccines. JAMA. 2021;325(13):1318-1320.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-416.
- Edara VV, Lai L, Sahoo M, Floyd K, Sibai M. Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B. 1.617. 1 variant. bioRxiv. 2021.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N Engl J Med. 2020;383(27):2603-2615.
- Tenforde MW, Olson SM, Self WH, Talbot HK, Lindsell CJ, Steingrub JS, et al. Effectiveness of pfizer-bioNTech and moderna vaccines against COVID-19 Among Hospitalized Adults Aged ≥65 Years - United States, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):674-679.
- Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 COVID-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021; 385(2 ):187-189.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet Lond Engl. 2021;397(10269):99-111.
- Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. Lancet. 2021;397(10285):1646-1657.
- Emary KRW, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet Lond Engl. 2021;397(10282):1351-1362.
- Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1885-1898.
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet Lond Engl. 2021;397(10275):671-681.
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S Vaccine against COVID-19. N Engl J Med. 2021.
- CanSinoBIO’s COVID-19 vaccine 65.7% effective in global trials, Pakistan official says | Reuters.2021.
- Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803-812.
- Wang G-L, Wang Z-Y, Duan L-J, Meng Q-C, Jiang M-D, Cao J, et al. Susceptibility of circulating SARS-CoV-2 variants to neutralization. N Engl J Med.2021;328(24):2354-2356.
- The Sinopharm COVID-19 vaccine: What you need to know.2021.
- Wang G-L, Wang Z-Y, Duan L-J, Meng Q-C, Jiang M-D, Cao J, et al. Susceptibility of circulating SARS-CoV-2 variants to neutralization. N Engl J Med 2021;328(24):2354-2356.
- Vaccine information, ICMR New delhi-Typography .2021.
- Sapkal GN, Yadav P, Ella R, Deshpande G, Sahay R, Gupta N, et al. Neutralization of UK-variant VUI-202012/01 with COVAXIN vaccinated human serum. BioRxiv. 2021.
- Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, et al. Efficacy of NVX-CoV2373 covid-19 vaccine against the B. 1.351 Variant. N Engl J Med. 2021;384(20):1899-1909.
- COVID-19 vaccine tracker. 2021.
- Covid-19 Vaccine Tracker:Latest Updates-The New York Times.2021.
- Biotechver.pdf.2021.
- Not much difference in post-vaccination infection rate for both Covaxin, Covishield: Data. India Today. 2021.
- Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, Klugar M. Prevalence of COVID-19 vaccine side effects among healthcare workers in the czech republic. J Clin Med. 2021;10(7):1428
- Shah ASV, Gribben C, Bishop J, Hanlon P, Caldwell D, Wood R, et al. Effect of vaccination on transmission of COVID-19: an observational study in healthcare workers and their households. medRxiv.2021.
- Kataria S, Sharma P, Deswal V, Kumar K, Singh M, Alam S, et al. A real world evaluation of the safety and immunogenicity of the covishield vaccine, ChAdOx1 nCoV- 19 corona virus vaccine (Recombinant) in health care workers (HCW) in national capital region (NCR) of India: A preliminary report. medRxiv. 2021.
- Singh B, Gandharava S, Gandharva R. COVID-19 vaccines and community immunity. Infect Dis Res Treat. 2021;2:5.
- Common COVID vaccine side effects in older adults.2021.
- Cirillo N. Reported orofacial adverse effects of COVID-19 vaccines: The knowns and the unknowns. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2021;50(4):424-427.
- Arvin AM, Fink K, Schmid MA, Cathcart A, Spreafico R, Havenar-Daughton C, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584(7821):353-363.
- CDC. CDC Works 24/7. Centers for disease control and prevention.2021.
Citation: Deka S, Chandolia B, Iram F, Hariharan N, Dubey A (2021) COVID-19 Vaccines: A Critical Appraisal. J Vaccines Vaccin. 12:460.
Copyright: © 2021 Deka S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

