Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2022) Volume 13, Issue 3
Control of Anthracnose Disease of Nigerian Native Strain Mango Fruits Using Hot Air and Hot Water Treatments
Oluwole Olakunle Oladele*, Oke Tolulope and Otori JenniferReceived: 12-Mar-2022, Manuscript No. JPPM-22-15839; Editor assigned: 18-Mar-2022, Pre QC No. JPPM-22-15839 (PQ); Reviewed: 01-Apr-2022, QC No. JPPM-22-15839; Revised: 06-Apr-2022, Manuscript No. JPPM-22-15839 (R); Published: 15-Apr-2022
Abstract
Control of anthracnose disease of mango fruits using hot air (HA) and hot water (HW) treatments was investigated. Nigerian native strain mango fruits of uniform size and color were selected, washed with clean water and disinfected for 10 minutes in 0.385% m/v of sodium hypochlorite and air-dried at 28 ± 2°C. The fruits were then inoculated with spore suspension (8.04 × 103 cells/ml) of C. gloesporioides. Artificially inoculated fruits in a separate experimental set up were then subjected to HA and HW treatments at 52°C, 55°C for 1, 3 and 5 minutes each before storage at 28 ± 2°C and 75 ± 5% relative humidity to determine disease severity while fruits that were not inoculated served as control. By day 20 in storage among the several temperature – time combinations experimented, only fruits treated at 52°C and 55°C for 3 minutes each with HA had a mean severity values of 1.40 ± 0.04 and 1.60 ± 0.25 respectively while fruits treated with HW at 52°C for 3 mins, 55°C for 1 min and 55°C for 5 mins had disease severity of 1.00 ± 0.00, 1.40 ± 0.40 and 1.50 ± 0.25 respectively, all indicating that the fruits were disease free. Consequently, these effective heat protocols could be applied as part of an integrated pesticide-free alternative for the control of mango anthracnose.
Keywords
Mangifera indica; Anthracnose; Inoculation; Heat treatments; Disease severity
Abbreviations
HA: Hot Air; HW: Hot Water; RH: Relative Humidity; MEA: Malt Extract Agar
Introduction
Mangoes are native to south Asia from where the “common mango” or “Indian mango”, Mangifera indica, has been distributed worldwide to become one of the most widely cultivated fruits in the tropics [1] and one of the highly consumed and popular fruits throughout the world and considered to be the oldest and best fruits in the world market [2]. They are highly nutritious fruits containing carbohydrates, proteins, fats, minerals, vitamins, particularly vitamin A (beta carotene), B1, B2 and vitamin C (ascorbic acid) [3]. In fact, one cup of sliced mangoes supplies 25% of the needed daily value of vitamin A, which promotes good eyesight. Higher intake of these vitamins and minerals are needed to reduce the higher percentage of night blindness and anemia prevalent among children. Diabetes has been treated with a drink made from the infusion of fresh mango leaves while dried mango seeds ground into flour is used to treat diarrhoea and throat disorders [4].
In spite of the growing worldwide demand for mango fruit, its production is affected by pre harvest and postharvest diseases, which reduce the fruit quality and cause severe losses, because they leave them as unmarketable fruits. The disease attack poses a serious threat because the postharvest life of mango fruits usually does not exceed 7 days and is limited by physiological deterioration of the fruit related to over ripening and by disease development leading to decay [5]. In fact, growing and marketing fresh mango fruits in Nigeria are threatened by post-harvest losses both in terms of quantity and quality. Among the major postharvest diseases of mango fruits are powdery mildew, die-back, anthracnose, stem end rot, transit rot, Phoma blight but anthracnose is the most serious and is widely distributed all over mango growing regions in the world [6] and causing considerable losses for the mango industry. The disease (anthracnose) is caused by Colletotrichum gloeosporioides or C. acutatum and is characterized by sunken black spots appearance on the surface of the fruit [7].
There are different strategies that can be employed for the management of postharvest diseases in mango fruits which include controlled environment, waxing, use of fungicides, handling and storage, irradiation and heat treatments. However, for fungicide and wax treatment, poisoning of the fruits due to chemical residue has been one of the limitations. Besides, concerns about environmental contamination and human health risks associated with fungicide residues periodically led to regulatory reviews and restrictions or cancellations. In addition, the wide spread and continuous use of these synthetic fungicides has led to the resistance of fungi which compromised the effectiveness of these fungicides [8]. In fact, the cost of developing new pesticides to overcome resistance developed by pathogens and the withdrawal of some chemical pesticides, such as benomyl and captan for control of postharvest diseases in the USA and ethylene dibromide for sterilization of Queen-land fruit fly in Australia, is a clear signal that new technology for control of plant diseases as an alternative to chemical fungicides is required. As a result, the use of non-chemical ecofriendly means of control such as heat treatments have emerged as viable alternatives.
Heat treatments have been used commercially to control fungal diseases and pest infestation since the first decades of the 20th century, when the effectiveness of hot water (44°C – 48°C) in controlling molds in citrus was reported [9]. Hot air has been used for both fungal and insect control and to study the response of commodities to high temperatures [1]. For instance, hot air treatment after inoculation had been discovered to increase the disease resistance of whole ‘Red Fuji apple fruit’ [10] and could completely control blue mold disease on the fruit while in 1992, for the first time, hot water treatment was employed to control rot on citrus fruits in Iran [11]. Also, immersing orange fruits of ‘clemenules’ variety in 60°C hot water for 1 minute decreased activity of green mold [12].
The efficacy of pre storage heat treatments has been well documented. Nevertheless, earlier authors [13,11] who carried out extensive studies on the effects of post-harvest heat treatments on fruits had concentrated so much on citruses and storage at cool temperatures. So far there has been no report on the effects of hot air and hot water treatments on disease severity caused by C. gloeosporioides in infected mango fruits during storage at 28 ± 2°C and 75 ± 5% relative humidity (RH). On the other hand, the geographical region can affect the response of mango cultivars to disease severity by C. gloeosporioides and heat treatments.
Therefore, the objectives of this study were (I) to explore the potential role of heat treatments and determine the specific temperatures – time combinations of HA and HW that can be used for the control of anthracnose disease of mango fruits during subsequent storage at 28 ± 2 ºC and 75 ± 5% (RH), and (II) to evaluate the effect of the HA and HW treatments on the sensory characteristics of the treated mango fruits.
Materials and Methods
Source of fruits
Mature, green and healthy mango fruits were harvested from an orchard in Oluwatuyi, Akure South, Nigeria (7.2146°N and 5.1641°E). 70 fruits of uniform size and maturity were selected. Before treatment, the fruits were washed with clean water, disinfected for 10 minutes in 0.385% m/v of sodium hypochlorite and allowed to air-dry at 28 ± 2°C.
Preparation and sterilization of culture medium
The culture medium used for isolation of fungi from the spoiled mango fruits and for preparation of pure cultures in the study was prepared by weighing 50 g of malt extract agar (MEA) into a conical flask to which was added 1 litre of water. The mixture was shaken together and sterilized in an autoclave at 121°C for 15 minutes. After sterilization, it was poured into oven sterilized Petri dishes and allowed to solidify.
Isolations from infected fruits
Isolation of the choice/test fungus (characterized by appearance of black spots) on the surface of spoiled mango fruits was made by cutting out the interface between the healthy and the disease tissue and placing pieces of the affected fruit rind without surface sterilization on plates of solidified malt extract agar. The plates were then incubated at 28 ± 2°C for 7 days. Sub culturing of the isolate was prepared by transferring agar cut with distinct mycelium to sterilized Petri dishes containing solidified MEA and then incubated at 28 ± 2°C until pure cultures were obtained. The resulting pure culture was then used for morphological characterizations of the isolate.
Morphological identification of the fungal isolate
After incubation, identification of the isolate was based mainly on the structural features as seen in the culture plates as well as microscopic characteristics. A drop of cotton-in-blue lactophenol solution was put on a slide. The isolate was placed on a slide. This was covered with a cover slip. Excess liquid was drained with filter paper and the isolate was examined under microscope. Examination was done with x40 objective for the presence and type of hyphae, mycelium whether clear or dark and spore morphology. The isolate was then identified using the text of [14].
Preparation of spore suspension
A ten day old agar slant culture of Collectotrichum gloeosporioides (the test isolate) on MEA was used to prepare spore suspension. Sterile water was poured into the slant and shaken vigorously to dislodge the spores from the vegetative hyphae. The wash water was collected in a sterilized beaker and serially diluted to × 103. 1 ml of the spore suspension was placed on the calibrated haematocytometer (Model 1280) slide and viewed under ×4 objective light binocular microscopes (Olympus-CH 11). The spore count was measured under four different fields and calculated as follows:
Cells/ml=(n) × 103
Where n=average cell count per square of the four corner squares counted
Pathogenicity test
Spore suspension of Collectotrichum gloeosporoides was used to inoculate fresh fruits 1 mm deep at the equator and incubated at 28 ± 2°C and 75 ± 5% relative humidity (RH) inside sterilized desiccators. The disease symptoms were noted and re-isolation from infected fruit tissue was performed on fresh sterile MEA plate and its cultural characteristics were compared with the original isolate.
Heat treatments
The suspension used to inoculate the mango fruits before treatment contained 8.04 × 103 spores m 1 for one of the test isolate. About 12 hours before treatment, the fruits were wounded at the equator with a sterilized syringe needle that was marked at 1 mm depth and injected with spore suspension of the fungus. The suspension was agitated before and during inoculation in order to maintain uniform spore distribution. Inoculated fruits were then separately placed in hot air oven at 52°C and 55°C for 1, 3 and 5 minutes each while inoculated fruits that were not treated served as control. In another experimental set up, inoculated fruits were also separately immersed in hot water in a water bath at 52°C and 55°C for 1, 3 and 5 minutes each. Each set up was in five replicates.
Assessment of root severity
All fruits were placed on sterilized Petri dishes for 1 hour and then stored in sterilized desiccators at 75 ± 5% RH and 28 ± 2°C and assessed daily for disease severity using the scale of [15] but with slight modification where 1=disease free, 2=slight rot/decay up to 10% of the fruit surface, 3=moderate rot/decay up to 25% of the fruit surface, and 4=severe rots/decay ≥ 35% of the fruit surface. Rot/decay was recognized by light black discoloration on the fruit or by appearance of mycelium on the fruit surface.
Sensory evaluation of mango fruits
Sensory evaluation of fruits which remained disease free after 20 days of storage following HA and HW treatments was done by an informal panel of ten judges. Fruit appearance and taste were observed. Fruits samples from effective treatments were labeled and laid out for the panel of judges to avoid biased judgment. Appearance index was determined by scoring graded fruit as very good/no sign of wilting on the entire fruit surface (4); good/no sign of wilting up to 90% of the fruit (3); showing sign of wilting up to 45% of the fruit (2) or wilted (1) [16]. Wiltness was evident by black colouration or appearance of mycelium on the fruit surface. Taste index was determined on hedonic scale of 1-5; where 1=Very Sweet; 2=Sweet; 3=Not Sweet; 4=No taste and 5=inedible [17].
Statistical analysis
The data obtained for disease severity and sensory attributes were subjected to analysis of variance (ANOVA) with five replicates using SPSS (version 20) software. The means, where significant were separated at 5% level of probability (P<0.05) using Duncan’s multiple range test.
Results
Effect of hot air and hot water treatments on disease severity of mango fruits
On day 5 of storage, results obtained on the effect of hot air (HA) treatment on mango fruits infected with Collectotrichum gloeosporioides and stored at 28 ± 2°C and 75 ± 5% RH showed that the disease severity of all the inoculated but treated fruits were not significantly different (p<0.05) from the control. Each treated and control fruits had a mean severity of 1.0 ± 0.00 which implied that the fruits were disease free. Similarly, the disease severity of both the treated fruits and the control with hot water (HW) treatment was equally 1.00 ± 0.00 which implied that the fruits were also disease free. As the storage duration progressed to day 10 with HA, only fruits treated at 52°C and 55°C for 3 minutes still maintained disease severity of 1.00 ± 0.00 while disease severities of fruits treated at 52°C for 1 and 5 minutes and the control were 1.80 ± 0.20, 1.60 ± 0.25 and 1.60 ± 0.25 respectively which implied that all were disease free. These values were however significantly different (p>0.05) from disease severity of fruits treated at 55°C for 1 and 5 minutes with respective disease severity of 2.20 ± 0.37 and 2.60 ± 0.40, showing slight decay. For HW, the disease severity of fruits treated at 52°C for 1, 3 and 5 minutes were 1.40 ± 0.25, 1.00 ± 0.00 and 1.20 ± 0.20 respectively while fruits treated at 55°C for 1, 3 and 5 minutes had 1.00 ± 0.00, 1.20 ± 0.20 and 1.20 ± 0.20 as their respective severities, indicating they were still all disease free and the values were significantly different (p>0.05) from the control with a disease severity of 2.00 ± 0.45 showing slight decay.
On day 15 of storage, fruits treated at 52°C and 55°C for 3 minutes with HA still maintained mean disease severity of 1.00 ± 00 which implied that they were disease free. However, they were significantly different (p>0.05) from fruits treated at 52°C for 1 and 5 minutes, 55°C for 1 and 5 minutes and the control with respective mean severity values of 3.80 ± 0.20, 3.00 ± 0.00, 4.0 ± 0.00, 4.0 ± 0.00 and 3.0 ± 0.00 indicating moderate to severe decay. For HW, the disease severity of fruits treated at 52 ºC for 1, 3 and 5 minutes were 1.80 ± 0.49, 1.00 ± 0.00 and 1.80 ± 0.49 respectively while fruits treated at 55 ºC for 1, 3 and 5 minutes had 1.00 ± 0.00, 1.40 ± 0.40 and 1.40 ± 0.40 as their respective severities which implied all were disease free. The values were not significantly different (p<0.05) from each other but significantly different (p>0.05) from the control with a severity of 2.40 ± 0.25, indicating slight decay. As storage duration progressed to day 20, fruits treated at 52°C and 55°C for 3 minutes with HA had a mean severity values of 1.40 ± 0.04 and 1.60 ± 0.25 respectively which were not significant different (p<0.05) from each other and indicating they were still disease free.
Nevertheless, the values were significantly different (p>0.05) from severities of fruits treated at 52°C and 55°C for 1 and 5 minutes, 55°C for 1 and 5 minutes and the control, each with severity value of 4.0 ± 0.00 showing severe rottenness. For HW, only fruits treated at 52°C for 3 minutes still maintained disease severity of 1.00 ± 0.00 which was not significant different (p<0.05) from severity values of fruits treated at 55°C for 1 minutes (1.40 ± 0.25) and 5 minutes (1.50 ± 0.37), implying they were still disease free. The values were however significantly different (p>0.05) from severity values of fruits treated at 52°C for 1 minute (2.60 ± 0.60), 52°C for 5 minutes (3.20 ± 0.20), 55°C for 3 minutes (2.20 ± 0.37) and the control (3.80 ± 0.20) showing slight to moderate decay.
Effect of hot air and hot water treatments on appearance and taste of treated mango fruits
Remarkably, fruits treated separately at 52°C and 55°C for 3 minutes each using HA were adjudged to be the best among all other treated fruits in terms of appearance and taste. By day 20, the mean appearance and taste of the treated fruits at 52°C for 3 mins were 3.40 ± 0.25 and 2.00 ± 0.45 respectively while appearance and taste of fruits treated at 55°C for 3 mins were 3.00 ± 0.25 and 2.60 ± 0.25 respectively all indicating good appearance and sweet taste of the treated fruits. Meanwhile, fruits treated with HW at 52°C for 3 mins, 55°C for 1 min and 55°C for 5 mins were adjudged to be the best in terms of appearance and taste. By day 20 in storage, the mean appearances of the treated fruits at 52°C for 3 minutes, 55°C for 1 min and 55°C for 5 mins were 4.00 ± 0.25, 3.50 ± 0.20 and 3.00 ± 0.56 respectively indicating good appearances while the mean taste values were 2.20 ± 0.20,2.60 ± 0.60 and 2.00 ± 0.00 indicating sweet tastes (Figures 1A and 1B).
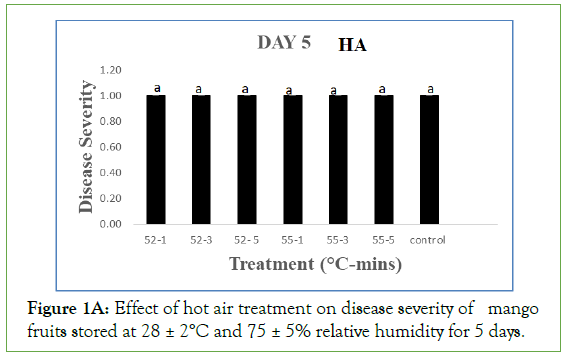
Figure 1A: Effect of hot air treatment on disease severity of mango fruits stored at 28 ± 2°C and 75 ± 5% relative humidity for 5 days.
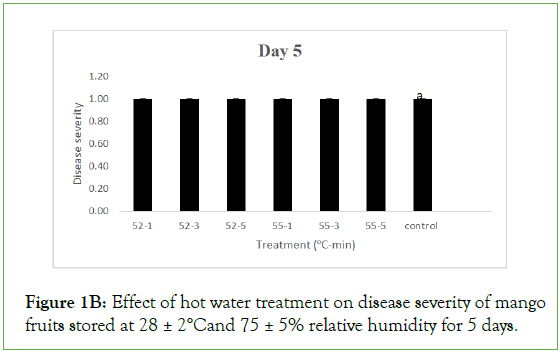
Figure 1B: Effect of hot water treatment on disease severity of mango fruits stored at 28 ± 2°Cand 75 ± 5% relative humidity for 5 days.
Same letters indicate no significant differences according to Duncan’s multiple range tests at the level p<0.05. The bars indicate standard errors (SE).
Note: 1=Disease free (Figures 2A and 2B).
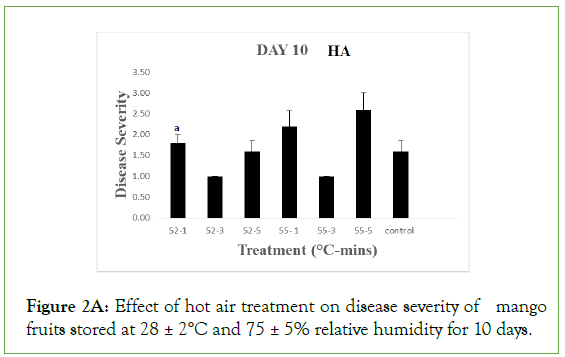
Figure 2A: Effect of hot air treatment on disease severity of mango fruits stored at 28 ± 2°C and 75 ± 5% relative humidity for 10 days.
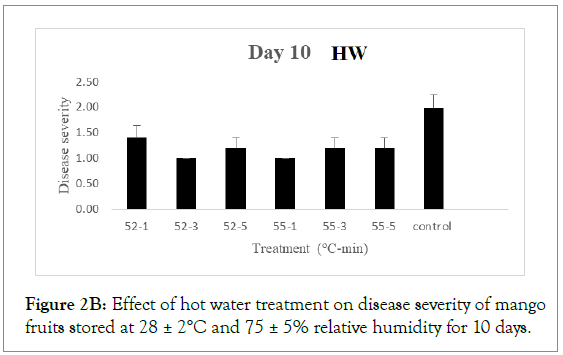
Figure 2B: Effect of hot water treatment on disease severity of mango fruits stored at 28 ± 2°C and 75 ± 5% relative humidity for 10 days.
Different letters indicate significant differences according to Duncan’s multiple range tests at the level p<0.05. The bars indicate standard errors (SE).
Note: 1=Disease free; 2=Slight decay (Figures 3A and 3B).
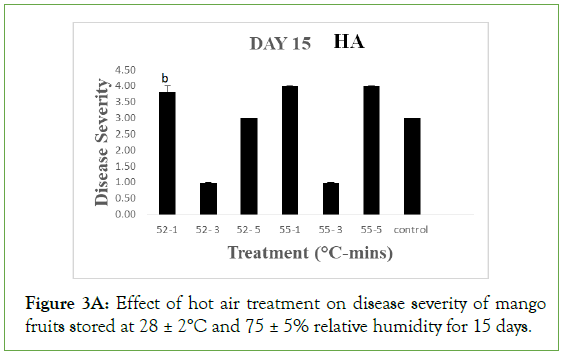
Figure 3A: Effect of hot air treatment on disease severity of mango fruits stored at 28 ± 2°C and 75 ± 5% relative humidity for 15 days.
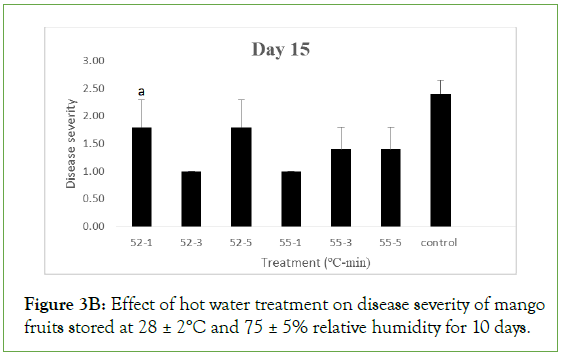
Figure 3B: Effect of hot water treatment on disease severity of mango fruits stored at 28 ± 2°C and 75 ± 5% relative humidity for 10 days.
Different letters indicate significant differences according to Duncan’s multiple range tests at the level p<0.05. The bars indicate standard errors (SE).
Note: 1=Disease free, 2=Slight decay, 3=Moderate decay, 4=Severe decay (Figures 4A and 4B).
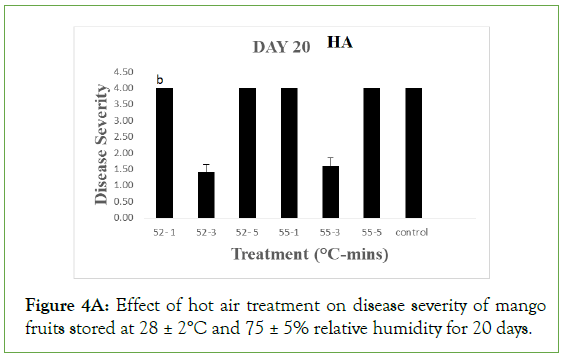
Figure 4A: Effect of hot air treatment on disease severity of mango fruits stored at 28 ± 2°C and 75 ± 5% relative humidity for 20 days.
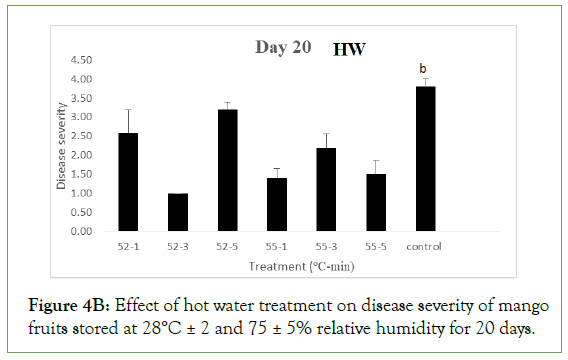
Figure 4B: Effect of hot water treatment on disease severity of mango fruits stored at 28°C ± 2 and 75 ± 5% relative humidity for 20 days.
Different letters indicate significant differences according to Duncan’s multiple range tests at the level p<0.05. The bars indicate standard errors (SE).
Note: 1=Disease Free, 2=Slight decay, 3=Moderate decay, 4=Severe decay (Tables 1A and 1B).
| Characteristics | 52ºC-3mins | 55ºC-3mins | Remarks |
|---|---|---|---|
| Fruit appearance | 3.40 ± 0.25 | 3.00 ± 0.25 | Good appearance |
| Fruit taste | 2.00 ± 0.45 | 2.60 ± 0.25 | Sweet taste |
Each value is a mean of 5 replicates.
Table 1A: Sensory attributes of mango fruits on day 20 of storage following the most effective hot air treatments.
| Characteristics | 52ºC-3mins | 55ºC-1min | 55ºC-5min | Remarks |
|---|---|---|---|---|
| Fruit appearance | 4.00 ± 0.25 | 3.50 ± 0.20 | 3.00 ± 0.56 | Good appearance |
| Fruit taste | 2.20 ± 0.20 | 2.60 ± 0.60 | 2.00 ± 0.00 | Sweet taste |
Each value is a mean of 5 replicates.
Table 1B: Sensory attributes of mango fruits on day 20 of storage following the most effective hot water treatments.
Discussion
Heat treatment is a safer way of preserving fruits from postharvest diseases because it leaves no residue on the fruit after treatment and it is environmentally safe unlike the use of fungicides. In facts, fruits treated with HA at 52°C and 55°C for 3 minutes each were disease free with no symptoms of spoilage. This showed that HA treatments at 52°C and 55°C for 3 minutes can be considered as the most effective treatment among all other HA temperature-time combinations while HW treatments at 52°C for 3 mins, 55°C for 1 min and 55°C for 5 mins among all the other HW temperature- time combinations effectively controlled anthracnose disease, showing no disease symptom on the mango fruits. These findings prove better than the earlier work of [18] who reported that mango fruits treated with vapour heat at 47°C for 15 minutes remained firm and with no sign of spoilage after 10 days in storage as against 20 days in storage observed in this work and the mango fruits still remained disease free (healthy) with HA at 52°C for 3 mins; 55°C for 3 mins and HW at 52°C for 3 mins, 55°C for 1 min and 55°C for 5 mins.
Similar results but in muskmelon were obtained by [19] who studied the effects of hot water dipping at 53°C for 3 mins. They found out that the treatments reduced decay caused by Trichothcium roseum, Alternaria alternata, Fusarium spp and Rhizopus stolonifer. The benefits of pre storage heat treatment on various horticultural produce especially in controlling pathological problems have been recorded [20]. HA treatments are beneficial means of controlling postharvest diseases and works by either killing the pathogen or its propagules or by suppressing its rate of development following treatments. In fact, forced hot air treatment appears to be as effective in controlling internal pest as vapour heat and provides better fruit quality [21] and indeed, the effective HA protocols used in this work proved not differently because the treated mango fruits showed no signs of spoilage after 20 days having good appearances and sweet tastes.
In the same vein, post-harvest hot water dips have been considered effective against mango anthracnose [22]. This was equally confirmed in this work as the effective HW protocols delayed anthracnose disease till after 20 days in storage as against earlier reports of [1] that anthracnose incidence in mango fruits treated at 52°C for 3 and 5 mins increased slightly after 6 days in storage and severe in mango fruits treated at 52°C for 1 min and 55°C for 1 min and 5 mins. Also, mango fruits treated by hot water at 52°C/5 mins and 52°C/10 mins did not show any remarkable symptoms of anthracnose infection at days 5, 10 and 15 in storage [2] and lastly, hot water controlled anthracnose caused by Collectotrichum gloesporioides on mango fruits at 52°C for 5 mins and 52°C for 10 mins and showed no remarkable symptoms of infection after 15 days of storage [6].
Consequently, the effective HW protocols observed in this work could be described as novel because of the extended shelf life over others, although factors that may account for different disparities according to [1] include varying tolerances of the cultivars to the treatment and sensitivities of the C. gloesporioides strains from different countries. The efficacy of HW treatments cannot but be connected with the fact that HW efficiently wash off dirt from the surface of the fruit, remove spores from wound and there is also the direct effect of heat on the pathogenic agent. In addition, the heat can melt the fruit wax, creating a mechanical barrier against pathogen penetration.
Conclusion
The limitations in using fungicide resources and probability of the development of resistance to the fungicides prompted us to search for viable alternatives that are non-chemical, ecofriendly, simple to implement, cheap and non-detrimental to human health. Consequently, we used hot air and hot water treatments at selected temperature and time combinations for the control of anthracnose disease in mango fruits and the study revealed that all the selected hot air and water treatments delayed anthracnose disease in the mango fruits. However, HA treatment at 52°C and 55°C for 3 minutes and HW treatment at 52°C for 3 mins, 55°C for 1 min and 55°C for 5 mins showed 100% effectiveness for the control of anthracnose disease on mango fruit for 20 days in storage at 28 ± 20C and 75 ± 5% RH and still maintained the sensory quality of the fruits. These effective heat protocols could be applied as part of an integrated pesticide-free alternative for the control of anthracnose decay by the fruit industry.
Acknowledgement
The authors thank Ojo Toyin and Gbolahan-Ayoade Elizabeth for technical assistance and useful contributions.
REFERENCES
- Shiesh C, Lin H. Effect of vapor heat and hot water treatments on disease incidence and quality of Taiwan native strain mango fruits. Int J Agric Biol. 2010; 12(5): 673-678.
- Obsa NA, Olyad GD, Tigist NT. Effect of hot water treatment on quality and incidence of postharvest disease of mango (Mangifera indicia L.) fruits. Asian J. Plant Sci. 2014; 13(2): 87-92.
- Lauricella M, Emanuele S, Calvaruso G, Giuliano M, D’Anneo A. Multifaceted health benefits of Mangifera indica L.(Mango): the inestimable value of orchards recently planted in Sicilian rural areas. Nutrients. 2017; 9(5): 525.
[Crossref] [Google Scholar] [Indexed]
- El-Salhy FT, Khafagy SA, Haggay LF. The changes that occur in mango fruits treated by irradiation and hot water during cold storage. J Appl Res. 2006; 2(11): 864-868.
- Kumah P, Appiah F, Opoku-Debrah JK. Effect of hot water treatment on quality and shelf-life of Keitt mango. ABJNA. 2011; 2(5): 806-817.
- Obsa NA, Olyad GD, Tigist NT. Effect of hot water treatment on quality and incidence of postharvest disease of mango (Mangifera indicia L.) fruits. Asian J Plant Sci. 2014; 13(2): 87-92.
- Afanador-Kafuri L, Minz D, Maymon M, Freeman S. Characterization of Colletotrichum isolates from tamarillo, passiflora, and mango in Colombia and identification of a unique species from the genus. Phytopathology. 2003; 93(5): 579-587.
[Crossref] [Google Scholar] [Indexed]
- Oladele OO, Fatukasi OI. Control of Postharvest Decay on Cashew Fruit (Anacardium Occidentale L.) with Aqueous Extract of Cashew Leaf. J Med Plant Res. 2018; 8.
- Escribano S, Mitcham EJ. Progress in heat treatments. Stewart Postharvest Review. 2014; 10(3): 1.
- Chen L, Jing W, Wang H, Chen YY, Tu K, Shao XF, et al. Effects of pre-storage hot air treatments on the post-harvest quality and blue mold control of´ red fuji´ apple fruit. InIV International Conference on Managing Quality in Chains-The Integrated View on Fruits and Vegetables Quality. 2006; 793-798.
- Fatemi S, Borji H. The effect of physical treatments on control of Penicillium digitatum decay orange cv. Valencia during storage period. African Journal of Agricultural Research. 2011; 6(26): 5757-5760.
- Montesinos-Herrero C, del Río MÁ, Pastor C, Brunetti O, Palou L. Evaluation of brief potassium sorbate dips to control postharvest Penicillium decay on major citrus species and cultivars. Postharvest Biol Technol. 2009; 52(1): 117-125.
- Inkha S, Boonyakiat D, Srichuwong S. Effect of heat treatment on green mold infection in tangerine fruit cv. Sai Num Pung. Chiang Mai Univ. J. Nat. Sci. 2009; 8(1): 85-92.
- Alexopoulos CJ, Mims CW. Introductory mycology. Jhon Wiley & Sons. 1979.
- Miller WR, McDonald RE, Sharp JL. Quality changes during storage and ripening ofTommy Atkins' mangos treated with heated forced air. HortScience. 1991; 26(4): 395-397.
- Oladele OO. Control of Rhizopus: Induced decay in watermelon (Citrullus lanatus L.) fruits using heat treatment. Braz. J. Biol. Sci. 2018; 5(10): 619-626.
- Lamond E. Laboratory method of sensory evaluation of foods. Canada Central Experimental Station, Canada. 1997; 31-35.
- Heather NW, Corcoran RJ, Kopittke RA. Hot air disinfestation of Australian ‘Kensington’mangoes against two fruit flies (Diptera: Tephritidae). Postharvest Biol Technol. 1997; 10(1): 99-105.
- Yuan L, Bi Y, Ge Y, Wang Y, Liu Y, Li G. Postharvest hot water dipping reduces decay by inducing disease resistance and maintaining firmness in muskmelon (Cucumis melo L.) fruit. Sci Hortic. 2013; 161: 101-110.
- Aborisade AT, Ajibade AA. Effect of prestorage curing on storage life, internal and external qualities of sweet orange (Citrus sinensis). Revista Brasileira de Fruticultura. 2010; 32: 910-915.
- Ornelas‐Paz JD, Yahia EM. Effect of the moisture content of forced hot air on the postharvest quality and bioactive compounds of mango fruit (Mangifera indica L. cv. Manila). J Sci Food Agricul. 2014; 94(6): 1078-1083.
[Crossref] [Google Scholar] [Indexed]
- Arauz LF. Mango anthracnose: Economic impact and current options for integrated managaement. Plant Disease. 2000; 84(6): 600-611.
[Crossref] [Google Scholar] [Indexed]
Citation: Oladele OO, Tolulope O, Jennifer O (2022) Control of Anthracnose Disease of Nigerian Native Strain Mango Fruits Using Hot Air and Hot Water Treatments. J Plant Pathol Microbiol. 13:604.
Copyright: © 2022 Oladele OO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

