Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2016) Volume 7, Issue 2
Compatibility: A Key To An Efficient Matrix Acidizing Fluid Design
Abstract
In this work four acidizing treatment systems have been formulated to treat various degrees of wellbore damages due to organic and inorganic materials, fines, mud-cake and other particles that block perforations and pore spaces around the wellbore. The treatment fluids are: clay stabilisation system, matrix stimulation system, non retarded mud acid system and oil-based dispersant fluid system. Emulsion break tests were conducted at test temperatures of 80°F, 150°F and 190°F. Clay stabilisation and matrix stimulation systems separated out of solution within the acceptable time of 10 minutes at all the test temperatures and showed no incompatibility. The rest of the treatment fluid systems separated after ten minutes. It was observed that, the higher the temperature, the faster the separation in most of the tests. It can be concluded that, different formation fluids react and behave differently with matrix acidizing treatment fluids and thus every treatment fluid would have to be tested for compatibility with formation fluid to ensure a successful stimulation.
Keywords: Matrix Acidizing, Compatibility, Emulsion break,Separation time, Temperature, Productivity
Introduction
Wellbore damage describes any restriction to flow from near-well reductions in flow capacity.These damages are as a result of reductions in near-well permeability caused by perforating debris or from the solids or mud filtrate invasion caused by the drilling process as well as certain production operation practices including well stimulation. The compatibility issues addressed in this study pertains to that between the reservoir fluids and stimulation fluids due to the tendency of these combinations to causing damage to the wellbore if their compatibility is not ensured. The study also addresses compatibility between the formulation fluids with the various additives as well as the acids used in preparing the stimulation/cleaning fluids.
The aim of the study is to ascertain the behaviour of the matrix acidizing fluid upon contact with formation fluids; as to whether there will be sludge or sediments formation or whether it will it cause increment in the fluids viscosity?. The sludge and sediments which may form can end up blocking pores around the near wellbore and impeding flow of reservoir fluid. Increase in viscosity will make it difficult for the fluid to be pumped out of the wellbore after the acidizing operation, thus, increasing pumping pressure.
Matrix acidizing consists of injecting acid solution into the formation at a pressure below the breakdown pressure to dissolve some of the acid-dissolvable minerals present in the rock with the primary aim of removing damages near wellbore, which restores the natural permeability and improves well productivity [1]. Matrix acidizing treatments can be aimed for wellbore cleaning purposes, damage removal from sandstone formation, production enhancement in carbonate formation, etc. [2]. The necessary requirement for the these aims to be achieved is that the damages to be treated must be dissolvable in the acidizing treatment fluids since certain damages cannot be dissolved by acids such as paraffin plugging.
The requirements to achieving a successful acidizing include a proper fluid selection, efficient design as well as proper execution of the job planned.
In most cases, specific design has to be employed for the prevailing reservoir/formation conditions in order to meet up with the success of the operation. Multiple fluids (fluid systems) compose of base fluids and additives and are selected on the basis of lithology, damage mechanism and well condition [3]. Condition such as high temperatures, high water cuts as well as deep vertical and long horizontal wells have to be treated taking into consideration the factors and required strategies necessary to achieve a successful matrix acidizing job which can increase production.
The fore-most thing to consider is the selection of acid type to use for each of the conditions stated above and when that is settled, the right type of additives which can perform and withstand the existing formation conditions are also looked at. Once the treatment is completed, the spent acid should be immediately produced back to minimise damage by the precipitation of reaction products [4]. Acid systems used for oil and gas well stimulation may be grouped into conventional acid systems and retarded acid systems (gelled acids, chemically retarded acids and emulsified acids). These acids differ in their characteristics and the choice of the acid and any additives for a given situation depends on the underground reservoir characteristics and the specific intention of the treatment, for example, near wellbore removal, dissolution of scale in fracture, etc. [5].
In this work, acids used are mineral acids (Hydrochloric acid-HCl), organic acids (Acetic acids) and acid mixtures were employed. Acid systems used for stimulation operations are grouped into conventional and retarded acid systems which include chemically retarded and gelled acid systems [6]. For this study, retarded acid systems have not been employed.
The major challenge in production enhancement fluid design is to come up with an economical fluid system which can improve oil recovery substantially without causing incompatibility upon contact with formation fluids and this is what this study seeks to establish. The tests carried out on the fluids include additives solubility/dispersability test, blend compatibility as well as emulsion break test.
Materials and Methods
The following chemicals and equipment are the materials employed in this study:
a) Crude oil from the Niger Delta region
b) Acids (HCl, HF, ABF, etc.), KCl, Diesel, Xylene, Mix water
c) Special additives comprising of Corrosion inhibitors, Surfactants, Mutual solvents, Iron Control agent, pH Control, Emulsifier, Non-Emulsifier, Penetration Aid and etc.
d) Hot water bath for temperatures, measuring cylinder, mixer, pipettes, beakers, etc.
The method used in this study is emulsion break test/analysis. Emulsion break test aims at determining the most effective fluid system (surface active agent with necessary concentration of other additives) that will prevent induced emulsions and sludge formation between the treatment fluid systems and the crude from the formation being treated [6]. Additives Solubility test was carried out by ensuring that all the necessary additives in the acid blend or mixture are compatible and there is no additive separation or precipitation. For this reason, the mixtures were prepared and observed standing for about an hour. The mixture is good and acceptable if no separation of additives occurs to adhere to the sides of the glass jar or settles at the sides or bottom.
Blend compatibility ensures effective combination of additives and their required concentrations which are necessary to prevent stable induced emulsions. The treatment and the formation fluids were mixed in the ratio of 1:1in a measuring cylinder, agitated thoroughly for a uniform mixture upon observation and allowed to stand for 10 minutes at temperatures of 80°F, 150°F and 190°F respectively. If separation of the mixtures occurs with the 10 minutes, the test is regarded as successful [7].
Recipe and formulation procedures
There are four different recipes used in formulating the four treatment fluid systems in this study. The formulation procedures have also been outlined for each treatment fluid (Table 1).
| Sn. No. | Chemical/Description | Concentration (1000 Gal) | Amount in 1000 Ml |
|---|---|---|---|
| 1 | Mix water | 995 Ml | |
| 2 | KCl (4 %) | 14.158 1b/bbl | 40.4 g |
| 3 | Surfactant | 0.5 % | 5 Ml |
Table 1: Recipe for clay stabilization system.
Fluid I (Clay stabilisation fluid): This formulation intends to be used for the stabilisation of clay content in the formation around the wellbore, in order not to result in any adverse reactions such as clay swellings and fines migration which can eventually block the formation pores and perforations. Emulsion break test results are given in Table 2.
| Composition | Temperature | Separation @ 10 minutes | Observation | Break | Colour | Interface | Sediment |
|---|---|---|---|---|---|---|---|
| Clay Stabilization+Crude (1:1) | 80 °F | 100% separation at 9mins:30secs | Dark brown homogenous mixture was formed upon agitation | Clean | Colourless acid fluid | Sharp | None |
| 150 °F | 100% separaion at 1min:26 secs | Dark brown homogenous mixture was formed upon agitation | Clean | Colourless acid fluid | Sharp | None | |
| 190 °F | 100% separation at 1min:33secs | Dark brown homogenous mixture was formed upon agitation | Clean | Colourless acid fluid | Sharp | None |
Table 2: Emulsion break analyses for clay stabilization + Crude (1:1) fluid systems.
Procedure:
a) The treatment fluid was prepared according to the various recipes in the table below.
b) A clear mixture solution was obtained.
c) Treatment fluid and the sample crude oil were mixed in ratio 1:1, thoroughly agitated, poured into graduated measuring cylinders and left to stand for observations at temperature of 80°F and repeated for 150°F and 190°F for the emulsion break analysis.
Fluid II (Matrix stimulation fluid): Fluid II was designed to depict matrix stimulation fluid capable of removing damages from formation matrix near wellbore. Table 3 shows the recipe used for the formulation. Emulsion break test was conducted for this formulation and sample crude and the results are presented in Table 4 for temperatures of 80°F, 150°F and 190°F respectively. It was observed from the fluid – crude mixture that, no sludge formation occurred and the additive did not separate out of the mixture. This proves to be good characteristics for any treatment fluid for stimulation purposes.
| Sn. | Chemical/Description | Concentration | Amount in 1000 Ml |
|---|---|---|---|
| 1 | Mix water | 998 Ml | |
| 2 | KCl (4 %) | 14.158 lb/bbl | 40.4 g |
| 3 | Corrosion Inhibitor | 0.2 % | 2 Ml |
Table 3: Matrix stimulation fluid recipe.
| Sn. | Chemical/Description | Concentration (Per 1000 Gal) | Amount in 200 ml |
|---|---|---|---|
| 1 | Mix water | 540 gals | 108 ml |
| 2 | HCl | 385 gals | 77 ml |
| 3 | ABF | 200 lbs | 4.8 g |
| 4 | Corrosion Inhibitor | 5 gals | 1ml |
| 5 | pH Control | 10 gals | 2 ml |
| 6 | Penetration Aid | 2 gals | 0.4 ml |
| 7 | Non-Emulsifier | 5 gals | 1 ml |
Table 4: Non-retarded mud acid fluid recipe.
Procedure:
a) The treatment fluid was prepared according to the recipe in Table 3.
b) A clear mixture solution was obtained.
c) Treatment fluid and the sample crude oil were mixed in ratio 1:1, thoroughly agitated, poured into graduated measuring cylinders and left to stand for observations at temperature of 80°F and repeated for 150°F and 190°F for the emulsion break test analysis.
Fluid III (Non-retarded mud acid): Fluid VII was formulated to be a fast reacting fluid and the constituents are HCl and Ammonium Bi- Fluoride which under goes reaction to generate HF in-situ to form the required mud acid. Ammonium Bi-Fluoride produces HF acid when mixed with HCl acid. The recipe for this formulation is given under Table 3. Emulsion break test was performed with this fluid and sample crude to establish compatibility. The results are given in Table 5. No sludge was observed to have been formed in the crude – fluid mixture at all the temperatures and the additives did not separate out from the fluid mixture or fluid – crude mixture which shows compatibility [8].
| Composition | Temperature | Separation @ 10 minutes | Observation | Break | Colour | Interface | Sediment |
|---|---|---|---|---|---|---|---|
| Oil-Based Dispersant + Crude 1:1 | 80 °F | No Separation at 10 mins | Dark brown homogenous mixture was formed upon agitation | None | Dark brown | None | None |
| 150 °F | No Separation at 10 mins | Dark brown homogenous mixture was formed upon agitation | None | Dark brown | None | None | |
| 190 °F | No Separation at 10 mins | Dark brown homogenous mixture was formed upon agitation | None | Dark brown | None | None |
Table 5: emulsion break analyses for oil-based dispersant + crude (1:1) fluid systems.
Procedure:
a) The Non Retarded Mud Acid treatment fluid was formulated according to the recipe in the Table 3.
b) 50 ml of Non Retarded Mud acid was mixed with 50 ml of emulsion fluid in a 100 ml glass measuring cylinder
c) The mixture was thoroughly agitated and left to stand for 10 minutes at 80°F and repeated at 150°F and 190°F respectively for the emulsion break analysis.
d) A clean glass rod was dipped into the emulsion fluid sample and rinsed under slow running tap water to determine the nature of the emulsion.
Fluid IV (Oil-based dispersant): This fluid system is intended to act as a dispersant for an oil-based, perforating/breakdown fluid for cleaning up invert oil based mud before stimulation treatments. The constituents of this fluid system are given in Table 6. It has been designed to be used on oil based muds such as invert emulsion mud which are usually used in drilling through shale’s and other water sensitive formations. This prevents the adverse effects of gummy, viscous emulsion created when common aqueous perforating and breakdown fluids contact invert muds. In fact it can be used as a cleanout fluid by circulating it at high pumping rate [5].
| Composition | Temperature | Separation @ 10 minutes | Observation | Break | Colour | Interface | Sediment |
|---|---|---|---|---|---|---|---|
| Non Retarded Mud Acid+Crude (1:1) | 80 °F | No separation at 10 mins. Only 30% of the acid fluid | Dark brown homogenous mixture was formed upon agitation | Not clean | Brown | Not too sharp | None |
| 150 °F | 100% separation at 7mins:13secs | Dark brown homogenous mixture was formed upon agitation | Not clean | Brown | Not too sharp | None | |
| 190 °F | 100% separation at 3mins:48secs | Dark brown homogenous mixture was formed upon agitation | Not clean | Brown | Not too sharp | None |
Table 6: Emulsion break analyses for non-retarded mud acid + crude (1:1) fluid systems.
This treatment fluid was used for emulsion break test with sample crude and the results are presented in Table 7. Also it can be said of the fluid and the fluid – crude mixture that, they respond favourably with temperature and are devoid of formation of sludge and sediments. No observation was recorded for additives separating out of the mixture and this shows compatibility.
| Sn. No. | Chemical/Description | Concentration (Per 1000 Gal) | Amount in 200 ml |
|---|---|---|---|
| 1 | Diesel | 668 gals | 137.6 ml |
| 2 | Xylene | 250 gals | 50 ml |
| 3 | Surfactant | 14 gals | 2.8 ml |
| 4 | Mutual Solvent | 67 gals | 13.4 ml |
Table 7: Oil-based dispersant fluid recipe.
Procedure:
a) Oil-based dispersant treatment fluid was formulated according to the recipe in Table 6.
b) 50 ml of oil-based dispersant was mixed with 50 ml of emulsion fluid in a 100 ml measuring cylinder
c) The mixture was thoroughly agitated and allowed to stand for 10 minutes at temperature of 80°F and repeated at 150°F and 190°F respectively for the emulsion break analyses.
Results
The following Tables 5-8 present the observations made during the test carried out with the various treatment fluids upon mixing with the formation fluids in a 1:1 ratio at 80°F temperature and repeated for 150°F and 190°F respectively.
| Composition | Temperature | Separation @ 10 minutes | Observation | Break | Colour | Interface | Sediment |
|---|---|---|---|---|---|---|---|
| Matrix Stimulation+Crude (1:1) | 80 °F | 100% separation at 1mins:40secs | Dark brown homogenous mixture was formed upon agitation | Clean | Colourless acid fluid | Sharp | None |
| 150 °F | 100% separation at 25 secs | Dark brown homogenous mixture was formed upon agitation | Clean | Colourless acid fluid | Sharp | None | |
| 190 °F | 100% separation at 18 secs | Dark brown homogenous mixture was formed upon agitation | Clean | Colourless acid fluid | None |
Table 8: Emulsion break analyses for matrix stimulation + crude (1:1) fluid systems.
Effective Fluid Systems
The influence of temperature on the separation time of the formulated fluids-crude mixture has been established. Normally it is expected that a good treatment fluid system will separate out from the formation crude faster and for the purpose of this test time limit of 10 minutes was observed for all the experiments carried out at the test temperatures. This is to enable the spent treatment fluid to be pumped out of the wellbore after the stimulation operation. Also, it will help the fluid not to react with formation fluid so as to become highly viscous and difficult to pump or to prevent any further reactions leading to precipitations, sediment and sludge formation which can end up blocking formation pores/perforations/wellbore to create additional damages.
In this study, any fluid system which could not separate out from the formation crude within 10 minutes is classified as incompatible due to the reasons given above. Any fluid system whose separation time falls within 10 minutes shows compatibility and can be used for well stimulation without any problems.
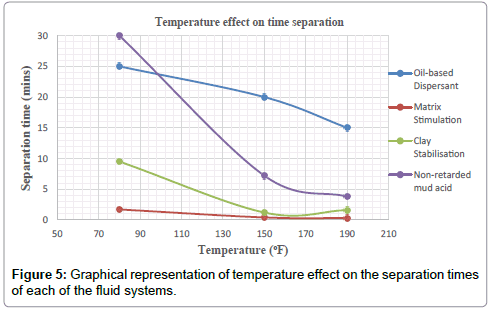
Plots of the various separation times achieved by each treatment fluid-crude mixture for the test temperatures of 80 °F, 150 °F and 190 °F are shown in the Figures.
Discussions on Emulsion
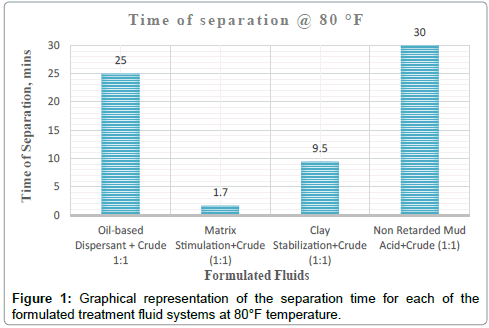
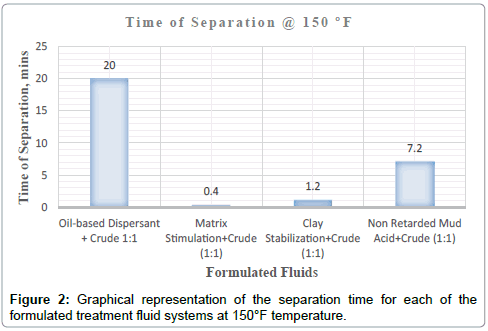
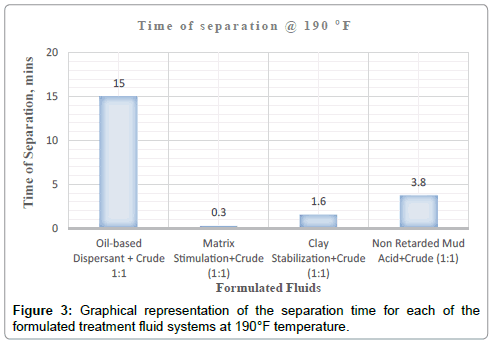
It can be deduced from Tables 5 and 6 and Figures 1-3 respectively that matrix stimulation fluid and clay stabilization fluids achieved a 100 % separation at 80°F, 150°F and 190°F within the ten (10) minutes. However, non-retarded mud acid system also achieved 100 % separation at 150°F and 190°F test temperatures as shown in Table 5 and Figures 2 and 3 respectively. This proves the fact that, non-retarded mud acid can be appropriate for high temperature conditions. All mixtures of the treatment fluids and crude exhibited clean break and sharp interface with the exception of non-retarded mud acid system as seen in Table 4. No sediments were observed for all the fluids mixtures with sample crudes. Different colourations were also observed for the various treatment fluid-crude mixtures as seen in Table 4.
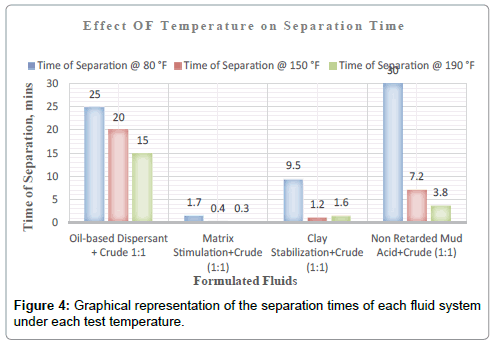
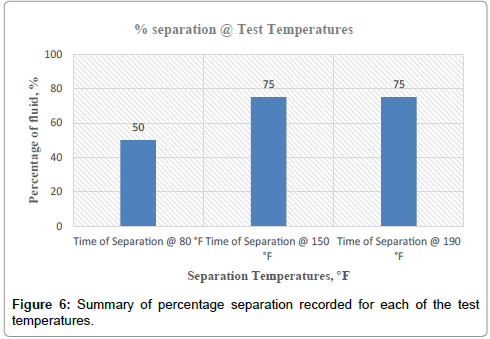
It can therefore be concluded that, matrix stimulation fluid and clay stabilization fluid systems exhibited good fluid-crude compatibility at all temperatures. However, Non Retarded mud acid should be used in areas of high temperatures for good results since increase in temperature improved its compatibility with formation crude. Figures 4 and 5 show the trend and give a summary of all treatment fluids-crude mixtures at the various test temperatures and their separation times. Table 9 shows the summary results of treatment fluid systems which successfully separated out of solution upon contact with the formation crude as well as those that could not. The percentage of treatment fluid separation at each test temperature is shown in Figure 6 where 75 % separation was recorded for 150°F and 190°F while 50 % separation was recorded for 80°F. On the whole, oil-based dispersant fluid system did not separate out from solution at all the test temperatures within the acceptable time limit and there is the tendency to react and form undesirable reactant products which can cause other wellbore problems.
| Fluid System | Time of Separation @ 80 °F | Time of Separation @ 150 °F | Time of Separation @ 190 °F |
|---|---|---|---|
| Oil-based Dispersant+Crude (1:1) | No separation | No separation | No separation |
| Matrix Stimulation+Crude (1:1) | Separation | Separation | Separation |
| Clay Stabilization+Crude (1:1) | Separation | Separation | Separation |
| Non-Retarded Mud Acid+Crude (1:1) | No separation | Separation | Separation |
| % separation | 50 % | 75 % | 75 % |
Table 9: Summary of fluid systems which achieved successful separation within the set test time of 10 minutes.
Conclusion
1. Clay stabilisation and matrix stimulation systems separated out of solution within the acceptable time of 10 minutes at all temperature and showed no incompatibility; no sediments, no sludge and additives dis not separate out of solution.
2. Generally temperature has influence on the time of separation of the fluid system from the crude; higher temperatures give faster separation which is a good indication of compatibility.
3. From the analyses, it can be seen that different treatment fluid systems react differently with crude hence the need to test for compatibility.
Acknowledgements
I would like to appreciate the University of Mines and Technology (UMaT) – Tarkwa and the World Bank Centre of Excellence in Oilfields Chemicals Research, IPS of the University of Port Harcourt for their supports.
References
- Aboud R, Smith K, Forero L, Kalfayan L (2007) Effective matrix acidizing in high-temperature environment. SPE Annual Technical Conference and exhibition, Anaheim, California, USA.
- Gdanski R (2009) Essential acidizing principles. Production Enhancement Handbook.
- Mahmoud EA (2013) Complete and cost-effective approach for diagnosing formation damage and performing successful stimulation operations. Journal of Petroleum Science Research 2: 65-74.
- Economides MJ, Hill AD, Ehlig-Economides C (1994) Petroleum production systems, (1stedn). Prentice Hall, Upper Saddle River, New Jersey, USA.
- Joel OF, Ajienka JA (2009) Identification of formation scales and design of compatible matrix acidizing fluid system. International Journal of Natural and Applied Sciences 5: 329-333.
- Joel OF (2010)Design and field application of drilling, cementing & stimulation fluids. Chi Ikoku Petroleum Engineering Series, IPS Publications.
- Cooley H, Dobson PF, Donnelly K, Feinstein LC, Fischer ML, et al. (2014) Advanced well stimulation technologies in California. California Council on Science and Technology, Lawrence Berkeley National Laboratory, Pacific Institute, USA.
- Halliburton (2008) N-Ver-Sperse O Dispersant
Copyright: © 2016 Dankwa OK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.