Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 0, Issue 0
Clinical Utility of Corona Virus Disease-19 Immunoglobulin G, M, Spike Protein, and Neutralizing Antibodies in Health, Disease and Post-Vaccination
Ernst J. Schaefer1,2, Florence Comite3,4, Latha Dulipsingh5,6, Maxine Lang1, Jessica Jimison7, Martin M. Grajower8, Nathan E. Lebowitz9, Andrew S. Geller1, Margaret R. Diffenderfer1, Lihong He1, Gary Breton1, Michael L. Dansinger1,2, Ben Saida10, Chong Yuan10 and R. Travis Wilkes112Department of Medicine, Tufts University School of Medicine, Boston, MA, USA
3Department of Clinical Affairs, Comite Center for Precision Medicine and Health, New York, USA
4Department of Medicine, Lenox Hill Hospital/Northwell, New York, NY, USA
5Department of Diabetes and Endocrinology, Saint Francis Hospital and Medical Center, Trinity Health of New England, Hartford, CT, USA
6Department of Medicine, University of Connecticut School of Medicine, Farmington, CT, USA
7Institute for Functional Medicine, Atkinson Family Practice, Amherst, MA, USA
8Department of Medicine, Albert Einstein College of Medicine, The Bronx, NY, USA
9Department of Cardiology, Advanced Cardiology Institute, Fort Lee, NJ, USA
10Research Department, Diazyme Laboratories, Inc., Poway, CA, USA
11Department of Medicine, Roper Saint Francis Physician Partners Primary Care, Mount Pleasant, SC, USA
Received: 23-Feb-2021 Published: 17-Mar-2021, DOI: 10.35248/2157-7560.21.s12.003
Abstract
Objective: About 80% of corona virus disease-19 (COVID-19) deaths due to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection occur in subject’s ≥ 65 years of age, even though subjects in this age group only account for about 10% of COVID-19 cases. Our objectives were to assess age effects and the clinical utility of COVID-19 antibody levels in health, disease, and post-vaccination.
Methods: We measured serum SARS-CoV-2 immunoglobulin M (IgM), IgG and neutralizing antibodies using immunoassay kits obtained from Diazyme (Poway, CA) and spike (S) protein antibodies using immunoassay kits obtained from Roche Diagnostics (Indianapolis, IN).
Results: In 79,005 subjects, IgG and IgM levels were positive (≥1.0 arbitrary units [AU]/mL) in 5.29% and 3.25% of subjects, respectively, with median IgG levels being 3.93AU/mL, 10.18 AU/mL, and 10.85 AU/mL in positive subjects <45 years, 45-64 years, and ≥65 years of age, respectively (p<0.0001). IgG antibody testing was found to be valuable for case finding in 1,111 exposed subjects with a wide variability in response. Persistently positive IgM levels were associated with chronic symptoms. Median IgG levels were 0.05 in 100 controls, 14.83 in 129 COVID-19 outpatients, and 30.61AU/mL in 49 COVID-19 hospitalized patients (p<0.0001). Neutralizing antibody levels correlated with IgG levels (r=0.875; p<0.0001). Post-vaccination (>2 weeks after second vaccine for Moderna, Pfizer, and AstraZeneca) in 105 subjects S protein antibody levels were all >250 U/mL and neutralizing antibodies were positive in all subjects except for 2 patients with chronic lymphocytic leukemia and 1 subject after the Johnson & Johnson vaccine. However, in all subjects, antibodies measured with the Diazyme IgG and IgM antibody and Roche total antibody levels were negative. S protein antibody levels were accurately assessed by fingerstick and micro-testing devices (Seventh Sense) in COVID-19 positive and negative subjects.
Conclusions: Our data indicate that: 1) IgG levels are significantly higher in positive older subjects than in younger positive subjects, possibly in order to compensate for the decreased cellular immunity observed in the elderly, 2) IgG levels are important for case finding and there is a wide variability in response, 3) persistently elevated IgM levels are associated with chronic symptoms, 4) IgG levels are correlated with neutralizing antibody levels, both of which are significantly elevated in hospitalized COVID-19 patients, and 5) S protein antibody levels are >250 U/mL after full vaccination except for those with leukemia, and can be accurately assessed by fingerstick or micro-testing technology.
Keywords
Corona Virus Disease-19 (COVID-19) antibody levels; Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2); Immunoglobulin G (IgG) antibody; Immunoglobulin M (IgM) antibody; Spike (S) protein antibody; Pandemic; Neutralizing antibody; Food and Drug Administration Emergency Use Authorization (FDA EUA); Naso-Pharyngeal (NP)
Introduction
Coronavirus disease-2019 (COVID-19), due to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, has caused a large world-wide pandemic with grave health and economic consequences. The diagnosis is made by SARS-CoV-2 RNA detection in Naso-Pharyngeal (NP) swabs, oro-pharyngeal, or nasal swabs usually within five days of exposure [1-5]. Up to 50% of SARS-CoV-2 positive patients can remain asymptomatic; however, such individuals can spread infections [6]. The average onset of symptoms after infection is about 5 days (range 2-14 days). Antibody testing has been reported to be useful for documenting exposure and potential immunity, as well as for case finding in family clusters and exposed individuals [7-14]. Moreover, treatment of hospitalized COVID-19 patients with convalescent plasma rich in antibodies or monoclinal antibodies may be useful in treating the disease [15-17].
In RNA positive subjects, IgM antibody levels are detectable within a median time of 5 days (range 3-7 days) of symptom onset and generally disappear over time, while IgG and neutralizing antibodies are detectable within a median time of 14 days (range 10-18 days) of symptom onset and generally persist for many months [7-14,18,19]. Similar results for SARS-CoV-2 antibodies have been obtained with chemiluminescence and enzyme linked immunoassays [7-14]. Levels of IgG antibodies have been shown to correlate with levels of neutralizing antibodies in serum with some assays, but not with others [18,19]. Fingerstick antibody testing with some lateral flow devices may be unreliable [20,21]. It has been reported by the Centers for Disease Control in the United States that about 80% of the total deaths attributed to SARS-CoV-2 occur in subjects ≥ 65 years of age, while this group only accounts for about 10% of the total cases [22]. Vaccines (Moderna and Pfizer) have been shown to be over 94% effective in preventing COVID-19 disease [23,24]. Our goals in the current investigation were to assess: 1) the effects of age on serum SARS-CoV-2 IgG and IgM antibody levels; 2) the clinical utility of such assays in case finding and symptom prediction; 3) the relationships of IgG and IgM antibody levels with neutralizing antibody levels; 4) the relationships of antibody levels in SARS- CoV-2 positive patients requiring hospitalization versus those not requiring hospitalization; and 5) antibody levels after vaccination.
Methods
Human subjects
A total of 79,005 subjects (median age 49.0 (IQR 35.0-69.0) years; 58.9% female, 18.2% ≥ 65 years of age) had serum samples submitted to our laboratory for the measurement of serum IgG levels. A subset of 62,048 subjects had samples submitted to our laboratory for the measurement of IgM levels. These subjects were assessed by healthcare providers in offices, clinics, hospitals, and at one meat packing plant (n=352). Samples were submitted to our laboratory between April 6th and September 1st, 2020. We also assessed data from samples collected by a healthcare provider from employees at a local meat packing plant in Massachusetts (n=217). In addition, we evaluated data on samples and information about clinical status submitted by healthcare providers for 534 outpatients and selected inpatients (median age 46 years, 51.2% female) from the Boston, Bronx, Manhattan, and northern New Jersey areas. For this latter research, subject data were extracted from medical records without name or identification number and were analyzed as anonymized data.
This type of research is exempted from requirement for human Institutional Review Board (IRB) approval as per exemption 4, as listed at https://grants.nih.gov/policy/humansubjects.htm and at the open education resource (OER) website for research involving human subjects. This exemption “involves the collection or study of data or specimens if publicly available or recorded such that subjects cannot be identified”. We had this designation and our research reviewed by the Advarra Institutional Review Board (IRB, Columbia, MD). They determined that “had the request for exempt determination been submitted prior to initiation of research activities, the research would have met the criteria for exemption from IRB under 45 CFR 46.104(d)” and, therefore, ruled that this research did not require IRB approval.
We measured all antibody levels (except for the S protein antibody) in 100 SARS-CoV-2 RNA negative control subjects, 129 SARS- CoV-2 RNA positive subjects not requiring hospitalization, and 49 SARS-CoV-2 RNA positive subjects requiring hospitalization (median age 48.9 years; 54.5% female; 85% Caucasian, 10 % Hispanic, and 7% African American). These subjects were enrolled in an IRB approved protocol at Trinity Health of New England (Hartford, CT, USA); all subjects provided verbal and written consent as previously described [16].
We evaluated serum levels of S protein antibody in venous and fingerstick and/or micro-testing samples obtained from 249 subjects (51% female, median age 33 years, of whom 26.9% had tested positive for SARS-CoV-2 RNA in nasal swab samples) as part of an IRB approved protocol. Self-collected fingerstick testing was done using McKesson #17 guage blade devices, with blood being collected in Becton Dickinson tubes. Self-collected blood was also obtained using microtesting devices (TAP II, Seventh Sense Biosystems, Medford, MA).
We have also measured serum SARS-CoV-2 IgG, IgM, S protein, neutralizing antibody levels in 103 subjects (64% female, median age 46 years, range 22-83 years) >2 weeks after the second dose of either the Moderna or the Pfizer vaccines in serum samples submitted to our laboratory by healthcare providers. We also measured levels in 36 of these 91 subjects 2-3 weeks after the first dose of their vaccine. Two of these subjects had chronic lymphocytic leukemia, one never treated, and the other receiving chemotherapy at the time of sampling. We also measured serum levels of the same antibodies in one 65 year old male subjects >2 weeks after the second dose of the AstraZeneca vaccine (study participant), and in one 52 year old female subjects >2 weeks after being vaccinated with a single dose as recommended of the Johnson & Johnson vaccine.
SARS-CoV-2 viral detection
Detection of SARS-CoV-2 RNA in NP or nasal swabs was performed using reverse transcriptase polymerase chain reaction methods with Thermo-Fisher TaqPath COVID-19 Combo kits (Waltham, MA). This assay targets a region in the N gene, a region in the spike glycoprotein or S gene, and a region in the ORF1 gene for SARS- CoV-2 RNA detection in swab samples. SARS-CoV-2 genomes are known to encode four main structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N). Positive values are those detected at a cycle threshold values of ≤ 37 cycles. Our modified version of this assay has received emergency use authorization (EUA) from the Food and Drug Administration (FDA) and was performed as previously described (3). Our assay was found to have 100% concordance in 100 positive and 100 negative samples when compared with another RNA assay from Viracor (Lee’s Summit, MO) as previously described [4].
SARS-CoV-2 IgG and IgM assays
The SARS-CoV-2 IgM and SARS-CoV-2 IgG chemiluminescence assays used were obtained from Diazyme Laboratories (Poway, CA) as previously described [8,12-14]. The assays use 2 recombinant antigens (full-length SARS-CoV-2 nucleocapsid protein and partial- length glycoprotein spike protein). The prediluted sample, buffer and magnetic microbeads coated with SARS-CoV-2 recombinant antigens are thoroughly mixed and incubated, forming immune- complexes. The precipitate is separated in a magnetic field and washed before N-(4-Aminobutyl)-N-ethyl-isoluminol labeled anti- human IgM or IgG antibodies are added and incubated to form additional complexes. After a second precipitation in a magnetic field and subsequent wash cycles, the Starter 1+2 is added to initiate a chemiluminescent reaction. The light signal is measured by a photomultiplier as relative light units (RLUs), which are proportional to the concentration of SARS-CoV-2 IgM or IgG present in the sample and are converted to arbitrary units or AU/ mL.
The SARS-CoV-2 IgG antibody test did not detect SARS-CoV-2 IgM antibodies, and the SARS-CoV-2 IgM antibody test did not detect SARS-CoV-2 IgG antibodies. For cross reactivity experiments, a total of 143 clinical samples were tested with both antibody assays. These samples were confirmed positive for antibodies for various viruses and bacteria: influenza virus type A, influenza virus type B, parainfluenza virus, respiratory syncytial virus, adenovirus, EBV NA IgG, EBV VCA IgM/IgG, Measles virus, CMV IgM/IgG, Varicella zoster virus, Mycoplasma Pneumoniae IgM/IgG, Chlamydia pneumoniae IgM/IgG, Candida albicans, ANA, HCoV-HKU1, HCoV-OC43, HCoV-NL63 and HCoV-229E. All 143 samples, however, were negative for SARS-CoV-2 IgG/IgM with DZ-Lite SARS-CoV-2 IgG/IgM CLIA kits. In addition, these assays were found to have no cross reactivity with antibodies for non-SARS- CoV-2 coronavirus strains HKU1, NL63, OC43, or 229E.
Multiple serum samples with IgM concentrations ranging from 0.86- 10.27 AU/mL and IgG concentrations ranging from 8.04–67.92 AU/mL had 0.1 mg/mL of the S protein and 0.1 mg/mL of the N protein added. After 10-minute incubations and remeasurements, mean IgM levels were reduced by 94.55% and mean IgG levels by 99.46%. These data confirmed that the antibodies measured in these assays are directed against the S and N proteins of SARS- CoV-2.
The specificity of the IgG assay for identifying 852 SARS-CoV-2 RNA negative outpatients was 97.40% when using IgG only; when used in combination with the IgM, the specificity was 96.00%. In 200 SARS-CoV-2 negative hospitalized patients, the specificity for diagnosing negative patients was 97.5% for the IgG assay alone and 96.5% for both IgM and IgG. In SARS-CoV-2 RNA positive patients (n=55), the sensitivity for detecting positive subjects for the IgG assay was 98.40% for those with symptoms ≥ 15 days; together with IgM it was 98.20%.
Positive values for both chemiluminescence assays are ≥ 1.0 AU/ mL, with linear and reproducible reportable ranges of 1.0–10.0 AU/mL for IgM and of 0.20–100.00 AU/mL for IgG. Linearity studies documented r2 values of 0.991 for both IgM and for IgG for actual values versus target values, with within- and between-run coefficients of variation based on 20 analyses at 4 concentration levels of 4.00% and 2.51% for IgM positive ( ≥ 1.0 AU/mL) control samples and 2.50% and 2.10% for IgG positive ( ≥ 1.0 AU/mL) control samples, respectively. Both these assays have received emergency use authorization (EUA) from the Food and Drug Administration (FDA).
Neutralizing antibody chemiluminescence assay
The SARS-CoV-2 neutralizing antibody assay utilized was obtained from Diazyme Laboratories (Poway, CA). This assay is a competitive chemiluminescence immunoassay based on the specific interaction between the SARS-CoV-2 spike protein Receptor Binding Domain (RBD) and the human angiotensin-converting enzyme 2 receptor (hACE2) on the surface of host cells. The assay has been previously described [14]. In the absence of SARS-CoV-2 neutralizing antibodies, hACE2 and RBD form complexes that generate a high chemiluminescent signal (measured in relative light units, RLU). In the presence of SARS-CoV-2 neutralizing antibodies originating from human serum or plasma, the interaction between hACE2 and RBD is compromised; and the chemiluminescent signal is reduced in a dose-dependent manner. The assay has been validated with a cell-based assay as previously described [25]. Serum samples (n=33) with neutralizing antibody values ≥ 2.60 AU/mL all showed >98.0% inhibition of viral infection in cell-based assay validation studies. The assay was documented to have no interfering substances and to be specific for SARS-CoV-2. In our laboratory this assay was found to have within- and between-run coefficients of variation of <4.0%, with positive value being ≥ 0.90 AU/mL and a linear range up to 30 AU/mL. This assay has been submitted for an FDA EUA.
Anti-SARS-CoV-2 S protein antibody assay
The anti-SARS-CoV-2 S protein antibody electro chemiluminescence immunoassay utilized was obtained from Roche Diagnostics (Indianapolis, IN) as described [26]. The assay has received FDA EUA status for the qualitative and semi quantitative detection of antibodies to the SARS-CoV-2 S protein RBD in human serum and plasma. The antigens within the reagent capture predominantly anti-SARS-CoV-2 IgG, but also anti-SARS-CoV-2 IgA and IgM. The analytical measuring interval was found to be from 0.40 units (U)/mL to 250 U/mL, with positive values being >0.80 U/mL. The negative percent agreement rate was 99.98% using 5991 serum samples obtained prior to October 1st, 2019. The positive percent agreement rate was 96.6% utilizing 1485 plasma samples from 331 symptomatic SARS-CoV-2 RNA positive patients [26]. The within and between run coefficients of variation of the assay were found to be <3.0% for positive samples with values >0.80 U/mL. We also used the qualitative Roche total antibody assay, but did not find it useful for assessing immunity after vaccination.
Statistical analysis
All statistical analyses were performed using R software, version 3.6 (R Foundation, Vienna, Austria). Categorical variables were expressed as frequencies and percentages, while continuous variables were expressed as median values with interquartile ranges (IQR, 25th–75th percentile values). The statistical significance of differences between groups was assessed using non-parametric Kruskal-Wallis analysis. Spearman correlation analyses were performed to assess interrelations of biochemical variables.
Results
Antibody testing in a reference laboratory population
Table 1 shows the results of testing 79,005 subjects for IgG and a subset of 62,048 subjects that also had serum IgM levels measured. IgG and IgM levels were positive ( ≥ 1.0 AU/mL) in 5.29% and 3.25% of these subjects, respectively. In positive subjects, median IgG levels were 3.93 AU/mL if <45 years, 10.18 AU/mL if 45- 64 years, and 10.85 AU/mL if ≥ 65 years (p<0.0001). Therefore, subjects in the ≥65-year age group and the 45-64-year group had more than two-fold higher median IgG levels than subjects <45 years of age. These findings were true for both females and males. In addition, females in the ≥65-year age group were significantly (p<0.0001) more likely to have positive IgM values than females in the <45-year age group. In addition to age effects, we also noted that men in the 45-64-year age group had significantly (p<0.001) higher IgG levels than their female counterparts.
| Age<45 Years | Age 45-64 Years | Age ≥ 65 Years | % Difference | |
|---|---|---|---|---|
| (n=33,847; 42.7%) | (n=32,347; 40.7%) | (n=13,164; 16.6%) | (Older vs. Younger) | |
| IgG ≥ 1.0 AU/mL | ||||
| Total positive, N (%) | 1,651 (4.90%) | 1,815 (5.63%)‡ | 735 (5.61%)‡ | 14.49 |
| Median value (IQR) | 3.93 (1.84-10.87) | 10.18 (2.63-35.51)‡ | 10.85 (2.68-47.71)‡ | 176.08 |
| Female positive subjects, N (%) | 892 (4.49%) | 972 (5.12%)‡ | 383 (5.31%)‡ | 18.26 |
| Median value (IQR), AU/mL | 3.81 (1.85-9.61) | 8.44 (2.20-27.38)‡ | 10.76 (3.38-40.45)‡ | 182.41 |
| %with IgG >20 AU/mL | 115 (0.58%) | 314 (1.65%)‡ | 151 (2.09%)‡ | 260.34 |
| Male positive subjects, N (%)† | 759 (5.50%) | 843 (6.38%) | 352 (5.98%) | 8.73 |
| Median value (IQR), AU/mL | 4.03 (1.81-12.14) | 12.24 (3.27-42.77)‡ † | 10.93 (2.14-52.05) | 171.22 |
| %with IgG >20 AU/mL | 131 (0.95%) | 353 (2.67%) | 149 (2.53%) | 166.32 |
| IgM ≥1.0 AU/mL | ||||
| Total positive, N (%) | 712 (2.78%) | 912 (3.53%)‡ | 390 (3.67%)‡ | 32.01 |
| Median values (IQR), AU/mL | 1.44 (1.14-2.11) | 1.65 (1.24-2.85) | 1.56 (1.22-2.45) | 8.33 |
| Female positive subjects, N (%) | 366 (2.39%) | 441 (2.85%) | 222 (3.76%)‡ | 57.32 |
| Median values (IQR), AU/mL | 1.35 (1.12-1.86) | 1.52 (1.18-2.51) | 1.48 (1.20-2.39) | 9.63 |
| Male positive subjects, N (%)† | 346 (3.38%) | 471 (4.54%) | 168 (3.56%) | 5.33 |
| Median values (IQR), AU/mL | 1.54 (1.17-2.32) † | 1.77 (1.28-3.07) † | 1.69 (1.27-2.58) † | 9.74 |
Note: *A total of 79,005 subjects, median age 45.0 years (IQR 30-55), 58.31 % female, had IgG values measured. A subset of 62,048 subjects (median age 45.0 years [IQR 31-54]; 58.53% female) had IgM values measured. Of these subjects, 89.42% had an IgG value <0.20 AU/mL; 3.53% had an IgG value 0.20-<0.50 AU/mL; 1.76% had an IgG value 0.50-<1.0 AU/mL; 3.75% had an IgG value 1.0-20.0 AU/mL; and 1.54% had an IgG value >20.0 AU/mL. For IgM, 96.75% had a value <1.0 AU/mL; 3.19% had a value of 1.0-10.0 AU/mL; and 0.06% had a value >10.0 AU/mL.
†Males of all ages had IgG and IgM values that were significantly higher than their female counterparts (P<0.01), especially in the 45-64 year old age group. The Spearman correlation coefficient between IgG and IgM for all subjects with values >1.0 AU/mL was r=0.433 (P<0.001).
‡P<0.0001 for age comparisons to <45-year age group. The percentage values represent a comparison between the age ≥ 65-year group and the <45-year age group.
Table 1: SARS-CoV-2 antibody levels by age and gender*.
Note: *A total of 79,005 subjects, median age 45.0 years (IQR 30-55), 58.31% female, had IgG values measured. A subset of 62,048 subjects (median age 45.0 years [IQR 31-54]; 58.53% female) had IgM values measured. Of these subjects, 89.42% had an IgG value <0.20 AU/mL; 3.53% had an IgG value 0.20-<0.50 AU/mL; 1.76% had an IgG value 0.50-<1.0 AU/mL; 3.75% had an IgG value 1.0-20.0 AU/mL; and 1.54% had an IgG value >20.0 AU/mL. For IgM, 96.75% had a value <1.0 AU/ mL; 3.19% had a value of 1.0-10.0 AU/mL; and 0.06% had a value >10.0 AU/mL.
†Males of all ages had IgG and IgM values that were significantly higher than their female counterparts (P<0.01), especially in the 45-64 year old age group. The Spearman correlation coefficient between IgG and IgM for all subjects with values >1.0 AU/mL was r=0.433 (P<0.001). ‡P<0.0001 for age comparisons to <45-year age group. The percentage values represent a comparison between the age ≥ 65-year group and the <45-year age group.
Studies in meat processing plants
In one study of 352 subjects from a meat packing plant in Nebraska, 19.0% had positive IgG values; and 15.3% had positive IgM values. In a separate analysis by one of our healthcare providers of 217 employees at a local meat processing plant in Massachusetts tested with NP swabs, 24.0% were RNA positive. When 41 of these 52 positive subjects were retested 2 weeks later, 73.2% still had positive NP swabs, 70.7% had positive IgG values, 9.8% had positive IgM values, and 63.4% had been symptomatic. Median IgG and IgM in all 41 subjects tested were 20.53 AU/mL and 0.54 AU/mL, respectively. As shown in Figure 1A, there was a very large variability in IgG response (range <0.20-117.7 AU/mL). In addition, there were 25 subjects that had prior RNA negative swab testing and requested antibody testing because of having significant symptoms and known exposure to subjects that had tested positive with RNA testing. Of these, 64.0% had positive IgG levels and 28.0% had positive IgM values, with all subjects in the latter group having persistent symptoms. Median IgG and IgM values in these positive subjects were 24.73 AU/mL and 1.31 AU/mL, respectively. These data clearly document the benefits of antibody testing for case finding in previously exposed subjects even with negative RNA tests.
Studies in healthcare provider’s office
Out of 388 outpatients that had antibody testing in a healthcare provider’s office (MMG) in the Riverdale area of the Bronx, NY, and 17.5% had positive IgG values with or without positive IgM values, while another 4.9% had borderline IgG values between 0.50-1.0 AU/mL. Of these latter subjects, 60.0% had been or were symptomatic. Of 10 subjects in the borderline category, 3 were previously RNA positive on swabs, and 6 had a history of definite exposure. This healthcare provider felt that IgG values between 0.50-1.0 AU/mL should be classified as borderline. His data justified this conclusion.
Antibody levels in individual family clusters and cases
Out of 154 outpatients in Manhattan and New Jersey that had NP swabs and antibodies assessed, 85.8% were negative for any evidence of SARS-CoV-2. The remaining 14.2% (n=22) were positive and of these subjects, 7 were carefully followed over time along with their family members, as well as 9 individual cases (total of 47 subjects). Many had the following symptoms: fever, chills, body aches, inability to sleep, fatigue, dry cough, loss of smell and taste, shortness of breath, and diarrhea. Three cases (all aged >80 years) had to be hospitalized, and two required being placed on ventilators, with one of these latter patients dying. The data that we tabulated clearly indicated that 1) antibody testing was valuable for finding additional cases in family studies (observed in all families); 2) patients can have positive RNA results for up to 6 weeks (observed in 5 cases); and 3) patients with persistent symptoms often have persistently elevated IgM levels (observed in 11 cases).
Antibody levels in RNA positive outpatients and inpatients
Data on serum IgG, IgM, and neutralizing antibody levels in 100 SARS-CoV-2 RNA negative control subjects, 129 SARS-CoV-2 RNA positive outpatients, and 49 SARS-CoV-2 RNA positive inpatients are shown in Table 2. All control subjects had negative antibody levels (<1.0 AU/mL). Median IgG levels were about 300- fold and 600-fold higher in outpatients and inpatients as compared to controls (both p<0.0001) (Figure 1). The wide variation in IgG response in outpatients is shown in Figure 1B. IgG values ranged from 1.03-200.0 AU/mL in outpatients and from 0.05-169.5 AU/ mL in inpatients, similar to what we observed in positive meat packing plant employees (Figure 1A). Median IgM levels were about 1.8-fold and 5-fold higher in outpatients and inpatients as compared to controls (both p<0.0001). IgM values ranged from 1.09-13.58 AU/mL in outpatients and from 0.46-18.82 AU/mL in inpatients. Median neutralizing antibody levels were about 12- fold and 24-fold higher in outpatients and inpatients as compared to controls (both p<0.0001). Neutralizing antibody values ranged from 1.09-13.58 AU/mL in outpatients and from 0.35-18.82 AU/ mL in inpatients. Median IgG, IgM, and neutralizing antibody levels were all significantly higher in positive patients than controls. Correlation matrix data for levels of antibodies are shown in Table 3. Neutralizing antibody levels correlated most significantly (p<0.0001) with IgG (r=0.875) and IgM (r=0.654), while IL-6 values correlated most strongly with hs-CRP values (r=0.740, p<0.0001).
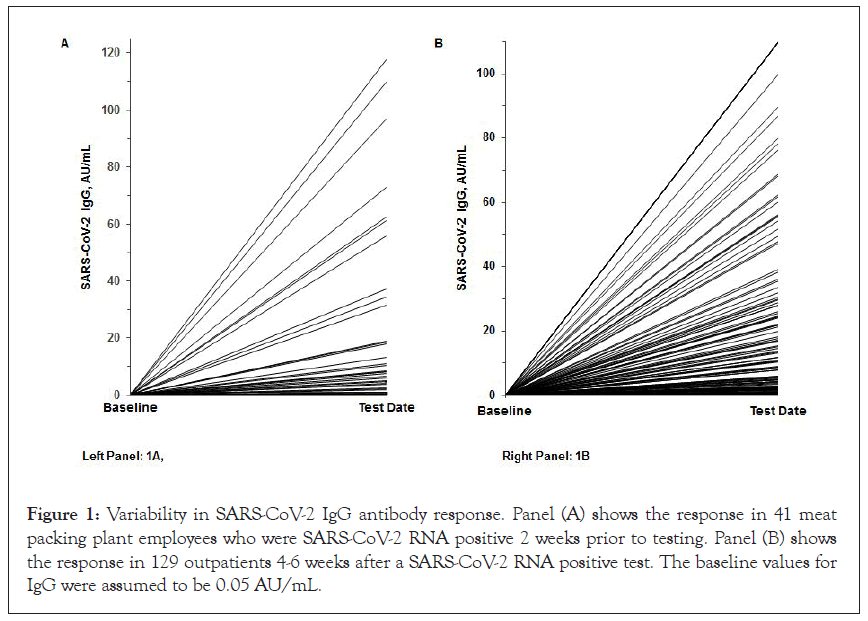
Figure 1: Variability in SARS-CoV-2 IgG antibody response. Panel (A) shows the response in 41 meat packing plant employees who were SARS-CoV-2 RNA positive 2 weeks prior to testing. Panel (B) shows the response in 129 outpatients 4-6 weeks after a SARS-CoV-2 RNA positive test. The baseline values for IgG were assumed to be 0.05 AU/mL.
| RNA Negative controls* | RNA Positive outpatients | RNA Positive inpatients | P value | |
|---|---|---|---|---|
| (n=100) | (n=129) | (n=49) | Trend† | |
| SARS-CoV-2 IgG, AU/mL | 0.05 (0.05‒0.05) | 12.20 (3.79‒35.20) | 30.61 (3.51‒75.02) | 3.48 × 10-40 |
| SARS-CoV-2 IgM, AU/mL | 0.43 (0.34‒0.54) | 0.76 (0.51‒1.33) | 2.16 (1.11‒3.56) | 7.50 × 10-24 |
| Neutralizing antibody, AU/mL‡ | 0.30 (0.20‒0.40) | 3.03 (2.04‒5.27) | 7.17 (4.00 ‒ 8.86) | 4.08 × 10-39 |
*Control subjects tested SARS-CoV-2 RNA not detected and SARS-CoV-2 IgG<0.2 AU/mL.
†P value for trend across the 3 subject groups. AU: Arbitrary Units; hs-CRP: high sensitivity C Reactive Protein.
Table 2: Antibody response in SARS-CoV-2 RNA positive outpatients and RNA positive inpatients vs. RNA negative control subjects.
| SARS-CoV-2 IgG | SARS-CoV-2 IgM | Neutralizing antibodies | |
|---|---|---|---|
| SARS-CoV-2 IgG | 1.000 | 0.642 (<1.00 × 10-12) |
0.872 (<1.00 × 10-12) |
| SARS-CoV-2 IgM | 0.642 (<1.00 × 10-12) |
1.000 | 0.646 (<1.00 × 10-12) |
| Neutralizing antibodies | 0.872 (<1.00 × 10-12) |
0.646 (<1.00 × 10-12) |
1.000 |
Note: Data are expressed as Spearman correlation coefficient r (P value). IgG: Immunoglobulin G; IgM: Immunoglobulin M.
Table 3: Spearman correlation coefficient matrix analysis of antibody response (n=278).
The use of fingerstick and micro device testing for s protein antibody assessment
We evaluated serum levels of S protein antibody in venous and fingerstick and/or micro-testing samples obtained from 249 subjects, of whom 26.9% had tested positive for SARS-CoV-2 RNA in nasal swab samples. S protein antibody were all < 0.4 U/mL in negative subjects and were all positive at >0.80 U/mL in positive patients. Subjects with values >250 AU/mL were assigned values of 260 AU/ mL, while those with values <0.40 AU/mL were assigned values of 0.20 AU/mL. Median values were 122.2 U/mL in positive subjects (range 13.6->250.0 U/mL). The overall correlation between venous blood samples and fingerstick values was 0.981 (p<0.0001), with no significant differences in mean values obtained (mean 24.66 U/ mL for venous blood and 24.59 AU/mL for fingerstick blood). It was documented that both venous and fingerstick samples could be stored at room temperature for up to 72 hours without spinning, and one still obtained virtually identical results as those obtained on samples spun right away and run then run immediately. In a subset analysis of 35 subjects, no significant differences were observed in values measured in blood obtained by finger stick or micro-testing devices. Moreover, there was 100% congruence with regard samples being positive or negative with regard to antibody levels <0.8 U/mL or ≥ 0.8 U/mL.
S Protein antibody assessment after vaccination
A total of 103 subjects were sampled >2 weeks after having received their second vaccination dose of either the Moderna or the Pfizer vaccine, with all subjects having S protein antibody levels of >250 U/ mL, and almost all subjects having negative IgG and IgM antibody levels using the Diazyme assays and negative total antibody levels using the Roche total antibody assay. However, all subjects had positive neutralizing antibody levels (median 11.22 AU/mL, range 1.18->30 AU/mL) >3 weeks after the second vaccination. In these same subjects, when sampled 2-3 weeks after their first dose of either the Moderna or Pfizer vaccines, their median S protein antibody levels were 104.2 U/mL (range <0.4->250 U/mL), and their median neutralizing antibody levels were 5.11 AU/mL (range <1.0-21.0 AU/mL). We did not see any significant differences in antibody responses comparing results obtained after either the Moderna or the Pfizer vaccines. However, there were 2 subjects sampled who had chronic lymphocytic leukemia. One subject on chemotherapy had negative S protein antibody and negative neutralizing antibody levels. The other subjects had a positive S protein antibody level of 7.5 U/mL and negative neutralizing antibody levels. In addition, there were one subject sampled >2weeks after both doses of the AstraZeneca vaccine and one subject sampled after ≥ 2 weeks after one dose as recommended of the Johnson and Johnson vaccine. The subject that received the AstraZeneca vaccine had an S protein value >250 U/mL and positive neutralizing antibody, while the subjects receiving the Johnson & Johnson vaccine had negative values for both antibodies tested.
Discussion
One of our goals in the current investigation was to assess the effects of age on serum SARS-CoV-2 IgG and IgM antibody levels. In a large number of outpatients with potential SARS-CoV-2 exposure, about 5% had positive IgG values and about 3% had positive IgM values. We noted significant age effects, with positive subject’s ≥ 65 years and those between 45-64 years of age having median IgG levels that were more than two-fold higher than their younger counterparts. We also noted a modest sex effect with men in the 45-64 year age group having significantly higher IgG levels than their female counterparts. It has been reported by the Centers for Disease Control and Prevention (CDC) that serum SARS- CoV-2 antibody levels were positive in 1.0-6.5% of 16,025 subjects in various parts of the United States, suggesting that infection rates were 6-24 times higher than reported at that time [27]. These percentages are similar to our data. Based on CDC data, over 95% of deaths from COVID occur in the >45-year age group, even though about 70% of the cases occur in those <45 years of age. The ≥ 65 years of age category accounts for ~10% of all SARS-CoV-2 cases and ~80% of SARS-CoV-2 mortality [22]. Possibly older subjects with positive antibody levels mount a greater IgG response in order to compensate for the decreased overall cellular immunity found in the elderly as compared to the young [28,29].
A second goal of our studies was to assess the clinical utility of antibody assays in case finding. We documented that antibody testing was valuable to identify cases and to ascertain potential exposure and level of immunity. The latter findings are relevant in the identification of potential convalescent plasma donors to assure sufficient IgG antibody levels. We have noted a high degree of variability in IgG antibody response in RNA positive patients. Laboratories that only report a positive or negative value do not detect this large variability. Moreover, only about 50% of RNA positive outpatients had IgG levels >6.5 AU/mL, sufficient to provide estimated antibody titers of >1:320, and only about one- third had plasma IgG levels >20 AU/mL, sufficient to provide estimated antibody titers >1:1000 [16].
In our individual and cluster studies, we have noted that antibody testing allows for the identification of exposed individuals, especially in those that were negative based on NP swab testing, usually ≥ 4 weeks following infection. Most of these Family Cluster and individual cases studies were carried by one of the co-authors (FC). She justifiably emphasized the value of both RNA and antibody testing in her practice. Her data clearly documented the benefits of semi-quantitative IgG and IgM testing for case finding in family clusters and exposed subjects who were RNA negative. Her data also indicated that RNA swabs can remain positive for up to 6 weeks, even though such patients may no longer be able to infect other people [30,31]. In her cluster and case data, we also clearly observed that long-term elevated IgM levels were often associated with persistent illness and symptoms. At the present time, very few healthcare providers are measuring COVID-19 antibody levels; instead, there has been a frenzy of nasal swab RNA testing [4,5]. Unfortunately, such testing in the United States has often been accompanied by a lack of public health measures or contact tracing to combat the spread of COVID-19. In our view antibody testing provides an excellent measure of prior exposure and potential immunity that has been greatly under-utilized in the United States [32].
A third goal of our studies was to investigate the relationships of neutralizing antibody levels with IgG and IgM antibody levels. We noted that both IgG and IgM were significantly correlated with neutralizing antibodies, but this relationship was strongest with IgG, consistent with prior reports [14,18,19]. A great advantage of the serum or plasma neutralizing assay we used in our studies was its ease of use on high through-put automated instruments and its reproducibility. Moreover, the results of this assay were found to be very highly correlated with results obtained using a cell-based assay [25].
A fourth goal of our studies was to examine the relationships of antibody levels in SARS-CoV-2 positive patients requiring hospitalization as compared to such subjects not requiring hospitalization. The highest median IgG, IgM, and neutralizing antibody levels that we observed were noted in hospitalized COVID-19 patients. As we have previously noted there was a high degree of variability in IgG response (Figure 1). Moreover, we observed a strong correlation between IgG levels and neutralizing antibody levels.
A final aspect of our studies was to evaluate the clinical utility of a newly released and approved semi quantitative S protein antibody assay from Roche Diagnostics. This assay was developed to specifically measure antibody levels to that portion of the S protein that the virus uses to bind to the angiotensin converting enzyme-2 receptor and to enter cells [33-35]. This region of the protein is presumably also the region induced by the mRNA SARS-CoV-2 Moderna and Pfizer vaccines. We found that this assay could readily be used to distinguish SARS-CoV-2 positive and negative subjects, even using fingerstick and microtesting devices. Moreover, the assay was found to be very useful for documenting immunity after vaccination with either the Moderna or Pfizer vaccines, with levels always being >250 U/ml three or more weeks after the second dose, but not after the first dose of vaccine. These data support the concept of using two doses 3-4 weeks apart as was done in the remarkably effective Moderna and Pfizer clinical vaccine trials [23,24].
What lessons can be learned from this pandemic that would be valuable for dealing with future pandemics? In our view, antibody assessment has been an underutilized tool in fighting the pandemic. Antibody measurements are valuable for case finding and for assessing immunity. Moreover, once such subjects have been identified, the evidence indicates that they are protected from further infection, and probably do not require vaccination. The CDC has emphasized that only RNA testing can be used for making the diagnosis of COVID-19 [36]. In our view, antibody testing is valuable for case finding and to assess for the level of immunity. Antibody testing should be more heavily utilized in future pandemics. At the present time in the United States subjects that have been fully vaccinated receive a certificate of vaccination. Moreover, our data indicate that after full vaccination with either the Moderna or Pfizer vaccines optimal levels of the S protein antibody (>250 U/L) are uniformly achieved. Public health authorities in the future might consider providing subjects with prior documented infection based on SARS-CoV-2 RNA testing and/or positive antibody testing with certification of prior infection. A significant limitation of our studies is the small numbers of human subjects sampled after vaccination. Some of the data presented here has been previously posted on a preprint server [37].
Conclusion
Our data are consistent with the following concepts:
1) SARS-CoV-2 IgG antibody levels are significantly higher in positive older subjects than in younger positive subjects, possibly to compensate for the decreased cellular immunity observed in older people.
2) Elevated SARS-CoV-2 IgG levels measurements are useful in identifying cases in exposed subjects and family clusters.
3) Elevated SARS-CoV-2 IgM levels are often associated with persistent COVID-19 symptoms and disease.
4) Serum SARS-CoV-2 IgG antibody levels are significantly correlated with neutralizing antibody levels and are especially elevated in hospitalized COVID-19 patients.
5) S protein antibody levels are >250 U/mL (optimal) at >2 weeks after vaccination.
Acknowledgements
We thank the laboratory staff at Boston Heart Diagnostics, Framingham, MA, and Diazyme Laboratories, Poway, CA, and the clinical staff at the Comite Center for Precision Medicine and Health, New York, NY, St. Francis Hospital/Trinity Health of New England, Hartford, CT, Atkinson Family Practice, Amherst, MA, Grajower Clinical Practice, the Bronx, NY, the Advanced Cardiology Institute, Fort Lee, NJ, and the Empower Diagnostics team especially Jennifer Griffith and Erin Jerger, for their efforts in carrying out the research on SARS-CoV-2 antibody testing.
Research support
This research was supported by Boston Heart Diagnostics, Clinical Enterprise Inc., Diazyme Laboratory, Seventh Sense Inc, and Trinity Health of New England.
Conflict of interest
There are no conflicts of interest to report other than that EJS, ML, ASG, MRD, LH, GB, and MLD are employees of Boston Heart Diagnostics, and that BS and CY are employees of Diazyme Laboratories.
REFERENCES
- Zhou XL, Yang XG, Wang B, Zhou P, Yang XL, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Schaefer EJ, Geller AS, Diffenderfer MR, Dulipsingh L, Wisotzkey J, Kleiboeker SB. Variability in coronavirus disease-2019 case, death, and testing rates in the United States and worldwide. medRxiv. 2020.
- Kleiboeker S, Cowden S, Grantham J, Nutt J, Tyler A, Berg A, et al. SARS-CoV-2 viral load assessment in respiratory samples. J Clin Virol. 2020;129:104439.
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): A review. JAMA. 2020;324:782-793.
- Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180.
- Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 2020;71:778-785.
- Lippi G, Salvagno GL, Pegoraro M, Militello V, Caloi C,Peretti A, et al. Assessment of immune response to SARS-CoV-2 with fully automated MAGLUMI 2019-nCoV IgG and IgM chemiluminescence immunoassays. Clin Chem Lab Med. 2020;58:1156-1159.
- Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, et al. Severe acute respiratory syndrome coronavirus 2−specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478-1488.
- Ren L, Zhang L, Chang D Wang J, Hu Y, Chen H, et al. The kinetics of humoral response and its relationship with the disease severity in COVID-19. Commun Biol. 2020;3:780.
- To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load, in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565-574.
- Suhandynata RT, Hoffman MA, Kelner MJ, McLawhon RW, Reed SL, Fitzgerald RL. Longitudinal monitoring of SARS-CoV-2 IgM and IgG seropositivity to detect COVID-19. J Appl Lab Med. 2020;5:908-920.
- Suhandynata RT, Hoffman MA, Kelner MJ, McLawhon RW, Reed SL, Fitzgerald RL. Multi-platform comparison of SARS-CoV-2 serology assays for the detection of COVID-19. J Appl Lab Med. 2020;5:1324-1336.
- Suhandynata RT, Hoffman MA, Huang D, Tran JT, Kelner M, Reed SL, et al. Commercial serology assays predict neutralization activity against SARS-CoV-2. Clin Chem. 2021; 30;67(2):404-414.
- Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA. 2020;324:460-470.
- Dulipsingh L, Ibrahim D, Schaefer EJ, Crowell R, Diffenderfer MR, Williams K, et al. SARS-CoV-2 serology and virology trends in donors and recipients of convalescent plasma. Transfus Apher Sci. 2020;59:102922.
- Cohen MS. Monoclonal antibodies to disrupt progression of early Covid-19 infection. N Engl J Med. 2021;384:289-291.
- Wajnberg A, Amanat F, Firpo A Altman DR, Bailey MJ, Mansour M, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227-1230.
- Tang MS, Case JB, Franks CE, Chen RE, Anderson NW, Henderson JP, et al. Association between SARS-CoV-2 neutralizing antibodies and commercial serological assays. Clin Chem. 2020; 66:12:1538–1547.
- Adams E, Ainsworth M, Anand R, Andersson MI, Auckland K, Baillie JK, et al. Evaluation of antibody testing for SARS-Cov-2 using ELISA and lateral flow immunoassays. MedRxiv. 2020.
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S. Cochrane COVID-19 Diagnostic Test Accuracy Group. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6.
- Centers for Disease Control and Prevention.COVID Data Tracker.2020.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S,et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615.
- COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384.
- Muruato AE, Fontes-Garfias CR, Ren P, Garcia-Blanco MA, Menachery VD, Xie X, et al. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat Commun. 2020;11(1):1-6.
- Roche Elecsys. RE Anti-SARS-CoV-2 S Package Insert. 2020.
- Havers FP, Reed C, Lim T, Montgomery JM, Klena JF. Hall AJ Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States. JAMA Intern Med. 2020.
- Wong GC, Strickland MC, Larbi A. Changes in T Cell Homeostasis and Vaccine Responses in Old Age. Vaccines for Older Adults: Current Practices and Future Opportunities. Interdiscip Top Gerontol Geriatr. 2020;43:36-55.
- Aspinall R. Age‐related changes in the function of T cells. Microsc Res Tech. 2003;62(6):508-513.
- Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Nat Med. 2020;183(7):1901-1912.
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nat. 2020;581(7809):465-469.
- Weitz JS, Beckett SJ, Coenen AR, Demory D, Dominguez-Mirazo M, Dushoff J, et al. Modeling shield immunity to reduce COVID-19 epidemic spread. Nat med. 2020;26(6):849-854.
- Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol.. 2020;5(4):562-569.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260-1263.
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al, Müller MA. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280.
- Jernigan DB, COVID C, Team R. Update: Public health response to the coronavirus disease 2019.
- Schaefer EJ, Comite F, Dulipsingh L, Lang M, Jimison J, Grajower MM, et al. Diffenderfer MR, He L, Breton G. Clinical utility of Corona Virus Disease-19 serum IgG, IgM, and neutralizing antibodies and inflammatory markers. medRxiv. 2021.
Citation: Schaefer EJ, Comite F, Dulipsingh L, Lang M, Jimison J, Grajower MM, et al. (2021) Clinical Utility of Corona Virus Disease-19 Immunoglobulin G, M, Spike Protein, and Neutralizing Antibodies in Health, Disease and Post-Vaccination. J Vaccines Vaccin. S12:003.
Copyright: © 2020 Schaefer EJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

