Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 4
Bone Marrow Biopsy Evaluation in Cases of Pancytopenia-An Institutional Experience
Pranati Mohanty1*, Ratiranjan Swain1, Sandhya Rani Sahoo1 and Sudha Sethy22Department of Clinical Hematology, SCB Medical College, Cuttack, Odisha, India
Received: 17-Jun-2020 Published: 21-Jul-2020, DOI: 10.35248/2155-9864.20.11.435
Abstract
Background: Pancytopenia is not a disease entity, but a triad of findings, resulting from various disease processes. It is characterized by anaemia, leucopenia and thrombocytopenia in the peripheral blood. Bone marrow examination is of utmost importance in evaluation of pancytopenia and thereby identification of disease process is helpful for appropriate management. Aims and Objectives: To analyse wide spectrum of haematological diseases giving rise to pancytopenia in bone marrow trephine biopsy. Materials and Methods: This was a prospective study conducted in the department of Pathology and Clinical Hematology, S.C.B. Medical College, Cuttack for a period of 2 years including 122 pancytopenia patients. Complete blood count including peripheral smear examination, followed by bone marrow aspiration (BMA) and trephine biopsy were carried out in all cases. Informations were compiled and analysed. Results: Among 122 patients studied, 63.1% were males and 36.9% females. Aplastic anaemia was the commonest cause of pancytopenia (51%) followed by megaloblastic anaemia (23%). Other causes include acute leukaemia 9%, MDS 6%, primary myelofibrosis 2%, lymphomatous infiltration in the marrow 2%, and multiple myeloma 3%. Metastatic deposit, tuberculosis, haemophagocytic syndrome and hypersplenism constituted 1% each. Conclusion: BMA and biopsy were diagnostic in all the cases of pancytopenia. Though in few cases BMA yields a dry tap & inconclusive coupled with bone marrow biopsy (BMB) it helps in defining diagnostic and therapeutic strategies in pancytopenia.
Introduction
Pancytopenia is not a disease entity, but a manifestation of a wide variety of disorders, which primarily or secondarily affects the bone marrow. There is reduction in all the three major cellular elements of blood. It is characterised by haemoglobin less than 13.5g/dl in males or 11.5g/dl in females, leukocyte count less than 4 × 109/l and platelet count less than 150 × 109/l [1].
The mechanisms contributing to pancytopenia include, decrease in haematopoitic cell production, marrow replacement by abnormal cells, suppression of marrow growth and differentiation, ineffective haematopoisis with cell death, defective cell formation, antibody mediated sequestration or destruction of cells in a hypertrophied and overactive reticuloendothelial system. The cause of pancytopenia may thus lie in the bone marrow, periphery or both [2].
The complete haematological work up with good clinical correlation is of utmost importance to evaluate the causes of pancytopenia and planning further investigations. The final interpretation requires the integration of peripheral blood findings, BMA and BMB evaluation together with supplementary tests such as immunophenotyping and immunohistochemistry (IHC). Cytogenetics and molecular studies also help in confirmation of diagnosis.
Marrow aspirate has been primarily utilized for cytomorphology. Trephine biopsy on the other hand, allows for assessment of the marrow’s overall cellularity, detection of focal lesions, and extent of infiltration by various pathologic entities [3]. Rationale for this study is to identify the diagnostic reliability of BMA and BMB in diagnosing various causes of pancytopenia.
Materials and Methods
This was a hospital based prospective cross sectional study conducted in the departments of Pathology and Clinical Haematology, SCB MCH, Cuttack, for a period of 2 years from August 2017-July 2019. Patients who fulfilled the criteria for pancytopenia were included in the study. Patients on chemotherapy and radiotherapy were excluded from the study. Detailed clinical history and physical examination was done in each case. All the patients were subjected to complete blood count (CBC) along with peripheral blood smear (PBS) examination and reticulocyte count. After taking informed consent BMA and BMB were then carried out from the posterior superior iliac crest using Salah’s needle and Jamshidi needle respectively. BMA smears and touch imprint smears of marrow biopsy were stained with Leishman stain. Hammersmith protocol was followed for fixation of trephine biopsy specimens (acetic acid-zinc formalin fixation for 12 to 24 hours) followed by decalcification (formic acid for 6 hours) [4] and then subjected to normal process of tissue processing and paraffin embedding. Tissue sections of size less than 4μ were cut and stained with hematoxylin and eosin. As and when required, BMA and BM biopsy were stained with Perl’s stain, periodic acid Schiff stain, reticulin and masson trichrome stain. Immunophenotyping of bone marrow aspirate was done for typing of leukemias. In case of metastatic and lymphomatous infiltration immunohistochemistry was also carried out for confirmation.
Detailed study of bone marrow examination was done and diagnoses were established in all the pancytopenia cases. All the relevant data were compiled and analysed for further investigation and planning.
Results
A total of 122 patients showing features pf pancytopenia were studied during this period. The age range was from 6 - 80 yrs with a mean age of 34.9yrs. Out of 122 cases 77 were males and 45 females with a male to female ratio of 1.7:1. It was observed that the incidence of pancytopenia was highest (22%) in the age group of 11-20yrs. In the biopsy evaluation, marrow was hypocellular in 51% cases, hypercellular in 38% and normocellular in 11% cases.
Aplastic anaemia was found to be the commonest cause of pancytopenia constituting 62(51%) cases followed by megaloblastic anemia 28(23%) cases. Other causes include acute leukemia 14 (11.5%) cases, myelodysplastic syndrome (MDS) 2(1.6%), primary myelofibrosis 2(1.6%), lymphomatous infiltration 2(1.6%), plasma cell myeloma 4(3.3%) and metastatic carcinoma 1(0.8%) case. Hemophagocytic lymphohistiocytosis (HLH) and hypersplenism were 2(1.6%) cases each. Tuberculosis was detected in one case (0.8%) Table 1.
| Causes | No. of cases | % |
|---|---|---|
| Aplastic anaemia | 62 | 51 |
| Megaloblastic anaemia | 28 | 23 |
| Acute leukaemia | 14 | |
| Multiple myeloma | 4 | 3.2 |
| NHL | 2 | 1.6 |
| MDS | 2 | 1.6 |
| Myelofibrosis | 2 | 1.6 |
| Metastatic carcinoma | 1 | 0.8 |
| Tuberculosis | 1 | 0.8 |
| Malaria | 2 | 1.6 |
| Hypersplenism | 2 | 1.6 |
| HLH | 2 | 1.6 |
| Total | 122 | 100 |
Table 1: Distribution of cases presenting with pancytopenia.
Out of 62 patients with aplastic anaemia 37 were male and 25 female. Most of the subjects (80%) had haemoglobin less than 7 gm/dl. Total count of white blood cells was 1000 to 2000/cu.mm in majority, only in 24% cases it was 3000 to 4000/cmm. Platelet count was below 40,000/cu.mm in the majority (52.6%). Both BMA and BMB showed decreased cellularity and increased fat cells (Fig-1). All these 62 cases were categorised as non-severe 35 (57%), severe 17(27%) and 10 (16%) as very severe according to Camitta et al, 1976 [5] and Bacigalupo et al, 1988 [6].
Megaloblastic anaemia was diagnosed in 28(23%) cases, of which 18(64%) were males and 10(36%) females. BMA and BMB showed hypercellularity in 95 % cases with characteristic megaloblastic erythropoiesis.
Acute leukemia was detected in 14 cases (11.5%), of which AML-M2 2 cases, APML 2, ALL-L1 6 cases and ALL-L2 were 4 cases. Immunophenotyping confirmed these as AML, APML, B-ALL and T-ALL. We found two cases (1.6%) of lymphomatous infiltration in the marrow, showing diffuse pattern. Both were cases of diffuse large B cell lymphoma, IHC showing CD 20 positivity (Fig-2).
Multiple myeloma was diagnosed in four cases (3.3%) of pancytopenia. BMA was inadequate in two cases, BMB was confirmatory showing sheets of myeloma cells with CD 138 positivity (Fig-3).
Eight cases of MDS were diagnosed in our study. BMA and BMB in each case showed abnormal erythropoiesis, myelopoiesis and megakaryopoisis showing dysplastic features along with abnormal localisation of immature precursors (ALIP) in BM biopsy.
Myelofibrosis were diagnosed in two cases (1.6%). Both had dry tap. BMB showed depression of erythropoiesis and myelopoiesis with areas showing extensive fibrosis (MF-2 & MF-3) on reticulin stain, (Fig-4). Both the cases had JAK2V617F mutation positive.
An interesting finding of bone marrow metastasis with advanced case of adenocarcinoma presenting as pancytopenia showed desmoplastic reaction apart from metastatic deposits in the marrow and on IHC showing CK 20 positivity on tumor cells thus confirming primary from GI tract (Fig-5). BMA was a failure in this case.
We also encountered a single case of tuberculous granuloma which was later confirmed by Ziehl Neelsen staining for AFB in BMB specimen. In this case BMA was dry tap but trephine biopsy revealed well defined granuloma (Fig-6).
Discussion
Pancytopenia often creates a diagnostic challenge for the clinicians. Identification of the accurate cause is crucial in the management of these patients. Bone marrow examination is essential in reaching the final diagnosis.
The present study conducted on 122 patients, showed a male preponderance M:F = 1.7:1. Age range was from 2-80 years. The mean age was 34.9 yrs. Highest number of patients were in the 2nd decade (22%) followed by 18% in the 5th decade. In a study conducted by Varma et al., on 62 patients with pancytopenia average age was 37.76 years [7]. The male: female ratio was 1.38:1.
The most common cause of pancytopenia was aplastic anemia (51%) followed by megaloblastic anemia (23%). Kumar et al, in their study detected 31.82% aplastic anemia and megaloblastic anemia in 24.03% [8]. However, in other Indian studies, megaloblastic anemia has been documented as the most common cause of pancytopenia [1,4,9]. This attributes to deficiency of vitamin B12 and folic acid which is very common. A comparison of various causes of pancytopenia outlined in different studies has been shown in Table 2.
| Study group | Country | Year | No. Of cases | Commonest cause (%) | 2nd most common cause (%) |
|---|---|---|---|---|---|
| Varma & Dash | India | 1992 | 202 | Aplastic anaemia (40.6) | Megaloblastic anaemia (23.3) |
| Tilak & Jain | India | 1999 | 77 | Megaloblastic anaemia (68) | Aplastic anaemia (7.7) |
| Kumar et al | India | 1999 | 166 | Aplastic anaemia (29.51) | Megaloblastic anaemia (22.3) |
| Khodke et al | India | 2000 | 50 | Megaloblastic anaemia (44) | Aplastic anaemia (14) |
| Gayathri et al | India | 2011 | 104 | Megaloblastic anaemia (74.04) | Aplastic anaemia (18.06) |
| Dasgupta et al | India | 2015 | 248 | Aplastic anaemia (33.47) | Megaloblastic anaemia (20.97) |
| Jyoti et al | India | 2019 | 166 | Hypersplenism (33.17) | Aplastic anaemia (13.9) |
| Present study | India | 2019 | 122 | Aplastic anaemia (51)) | Megaloblastic anaemia (23) |
Table 2: Common causes of pancytopenia in different study groups.
Other causes were acute leukemia (11.5%), MDS (1.6%), multiple myeloma (3.3%), lymphoma (1.6%), myelofibrosis (1.6%), metastatic carcinoma (0.8%). Infective causes diagnosed were tuberculosis (0.8%) and HLH (1.6%). Hypersplenism was detected in 1.6%.
Aplastic anaemia in this study showed a male preponderance with a M:F ratio 1.5:1 similar to other studies i.e 1.25:1 by Biswajit et al [10]. It was also the commonest cause of pancytopenia in the paediatric group (<14years) constituting 56%. Same observation was also noted by Zeb Jan et al. BMB revealed marked hypocellularity with predominance of fat spaces and focal haematopoietic activity (hot spots). There was no dysplasia or increased fibrosis in the reticulin stain. Depending on BM cellularity, neutrophil count, platelet count and reticulocyte count we found 57% cases were non-severe, 27% as severe and 10% cases were very severe types [5,6]. Biswajit et al reported 44.74% as non-severe, 34.21% severe and 21.05% with very severe disease [10].
Megaloblastic anemia was the second most common cause of pancytopenia in our study (23%). Many Indian studies reported high incidence 74.04% by Gayathri and Rao et al [1], 72% by Khunger JM et al [9], 68% by Tilak V et al [4]. Low incidence is also reported by Jyoti SK et al i.e 9% [11]. The mean age was found to be 28 years with a M:F ratio of 1.3:1. BMB revealed hypercellular marrow with megaloblastic erythropoiesis and reversal of M:E ratio. Vitamin B12 deficiency was detected in 65% patients and folate deficiency in 10%.
Acute leukemia constituted 11.5% of causes of pancytopenia, of which ALL-L1 were 6 cases, ALL-L2 4 cases along with APML 2 cases and AML-M2 2 cases. All these cases were confirmed by immunophenotyping from BMA as B-ALL, T-ALL, APML and AML respectively. In a study by Aziz et al acute leukemia constituted 10 % of cases of pancytopenia [12]. Kumar R et al. reported 5 cases of ALL, 13 cases of AML, 2 cases of hairy cell leukemia out of 166 cases of pancytopenia, over a 6-year study period [8]. Khodke et al. reported a single case of AML-M2 out of 50 cases of pancytopenia [2].
NHL infiltrating the marrow was noted in two cases (1.6%) in our study, both were follow up cases of DLBCL. Tumour cells showed CD20 positive on IHC (fig- 2). We have observed that NHL infiltrating the marrow is more common than Hodgkin’s lymphoma. Jyoti et al has reported 4 cases (2.4%) of NHL with bone marrow involvement [11].
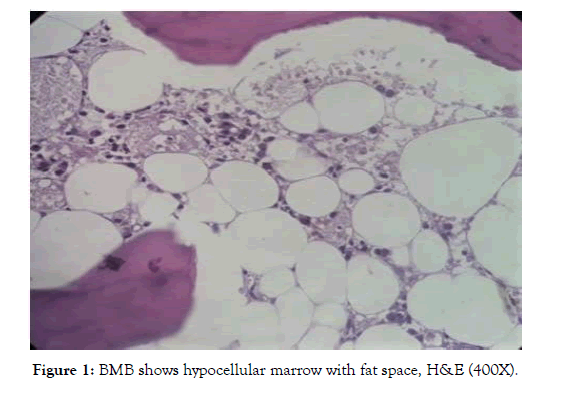
Figure 1: BMB shows hypocellular marrow with fat space, H&E (400X).
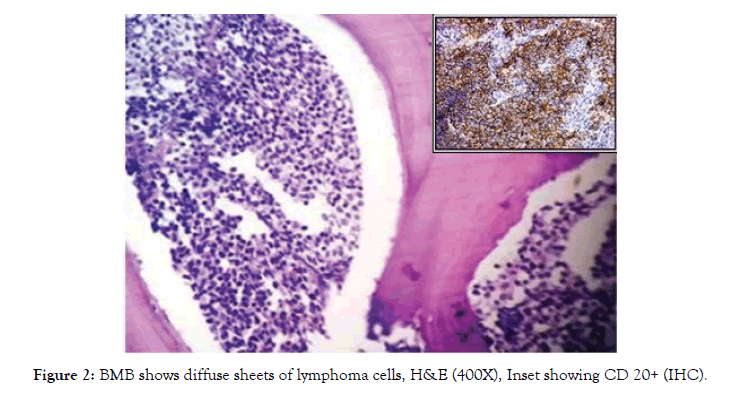
Figure 2: BMB shows diffuse sheets of lymphoma cells, H&E (400X), Inset showing CD 20+ (IHC).
Multiple myeloma in our study was found in 3.3% cases. IHC staining for CD138 confirmed the myeloma cells in sheets (fig- 3) its incidence varies in various studies. Gayathri and Rao reported 0.96% of MM in their series of 104 patients with pancytopenia [1]. However, a higher incidence (4%) of MM was reported by Khodke et al, Tilak V et al reported 3.9% and Kumar et al reported 3% of the total pancytopenia cases [2,4,8].
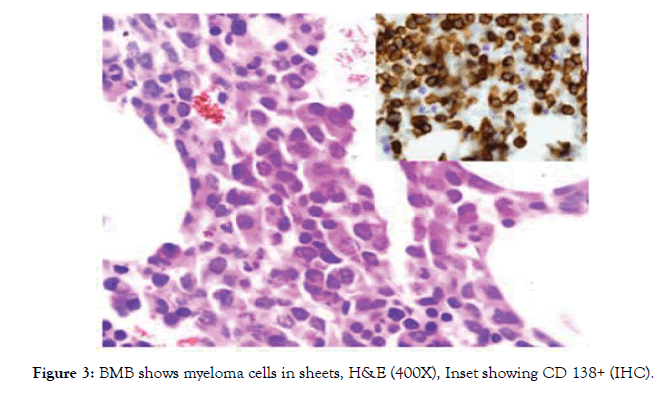
Figure 3: BMB shows myeloma cells in sheets, H&E (400X), Inset showing CD 138+ (IHC).
Two cases of myelodysplastic syndrome (MDS) were detected in our study constituting 1.6%. Both of them were elderly patients in the 6th decade. Dysplastic changes were noted in BMB in all the series. MDS is being reported by various studies 2.4% [11] and 2.42% by Dasgupta et al [13] and 18% by Devi PM et al [14]. It should be kept in mind as a possibility in elderly patients with pancytopenia.
Two cases of myelofibrosis (1.6%) were diagnosed in our study. BMA was dry tap, confirmed as fibrotic phase by trephine biopsy. Reticulin stain showed grade 2 & grade 3 fibrosis (fig- 4). JAK 2 mutation was positive in both. Literature reveals myelofibrosis in pancytopenia in studies done by Tilak V et al (1 of 77 cases) and Khunger JM et al (2 of 200 cases).
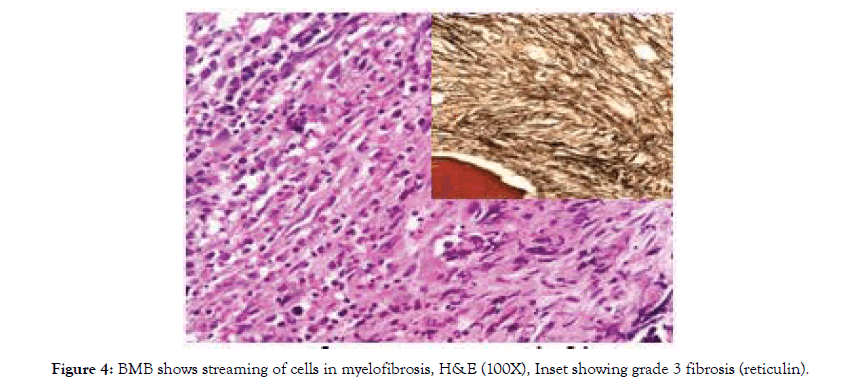
Figure 4: BMB shows streaming of cells in myelofibrosis, H&E (100X), Inset showing grade 3 fibrosis (reticulin).
Metastatic adenocarcinoma was detected in one case (0.8%). BMA was inconclusive, biopsy was confirmatory. Tumour cells were CK 20 positive pointing to a primary from G.I. tract (fig- 5). Dasgupta et al reported 4 cases (1.61%) of metastatic carcinoma in their study [13].
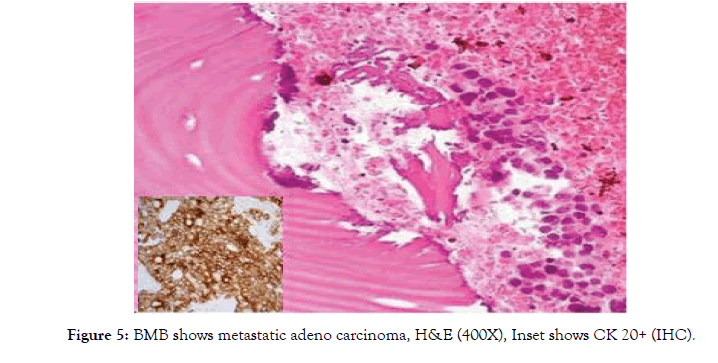
Figure 5: BMB shows metastatic adeno carcinoma, H&E (400X), Inset shows CK 20+ (IHC).
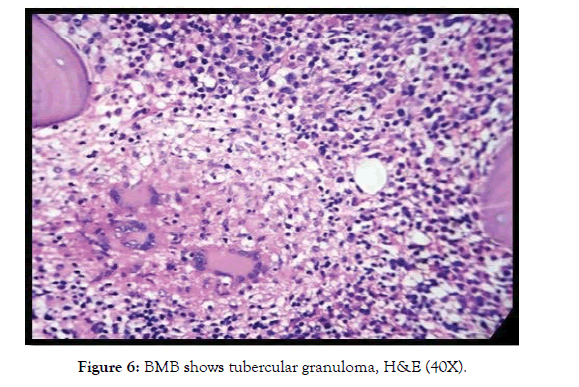
Figure 6: BMB shows tubercular granuloma, H&E (40X).
Infection induced pancytopenia was also encountered in our study. Disseminated tuberculosis in one case (0.8%), malaria in two cases (1.6%) and HLH (hemophagocytic lymphohistiocytosis) two cases (1.6%) secondary to E. B. Virus were detected. Infections accounting for 25.6% of causes of pancytopenia was reported by Jain A et al [15]. Tuberculosis accounted for 12 (4.84%) cases in a study by Dasgupta S et al [13]. We found BMA was a failure in which case well-formed granuloma was detected in BMB. P.C.Toi et al. observed that BMB along with good imprint alone is sufficient in diagnosis of granulomas [16]. TB should be considered as a differential diagnosis in patients presenting with unexplained fever, weight loss with pancytopenia. Degree of pancytopenia is affected more by duration of infection than by its severity [17]. Leishmaniasis was detected in 34 cases (13.71%) by Dasgupta et al. BM showed both intracellular and extracellular amastigote forms of leishmania donovani apart from increased plasma cells [13]. Several other infectious causes of pancytopenia reported by Jyoti et al are Hepatitis B, HIV and enteric fever [11]. Gayathri et al has encountered 2 cases of malaria in their study constituting 1.93% of total cases as compared to incidences by Khunger et al (1%), Tilak V et al (3.9%) and by Kumar et al (3%) [1,4,8,9].
Hypersplenism constituted 1.6% of total cases of pancytopenia in this study. BMB showed hypercellular marrow with trilineage hyperplasia. It was the commonest cause (33.17%) in a study by Jyoti et al [11]. Similar observation was also noticed by Jain et al who opined that increase in incidence of hypersplenism is due to chronic liver disease and infectious diseases in India, most commonly falciparum malaria [15].
BMA and biopsy both are equally useful in evaluation of pancytopenia. As cytologic details are better appreciated in aspirate smears, whereas biopsy sections are mainly helpful for measurement of cellularity, fibrosis, marrow infiltration, metastatic deposits along with bone marrow architecture and stromal changes like marrow necrosis, gelatinous transformation etc. So also, detection of granuloma is usually a failure in BMA, is confirmed in trephine biopsy as agreed by several authors.
Tilak and Jain had attributed the variations in the frequency of various etiologies causing pancytopenia to numerous factors such as differences in diagnostic criteria, study period, laboratory investigations used, geographic area, genetic differences in the study population and varying exposure to cytotoxic agents [4].
In the present study, BMA was dry tap in 5 cases (4.09%). All these were metastatic carcinoma, lymphoma infiltration, myelofibrosis in fibrotic stage and granuloma that were confirmed by BMB. Similar observation was also noticed by other authors like Navone et al [18] and Pandya et al [19] who found 100% abnormal biopsy in dry tap cases. But study done by Engeset et al [20] and Humphries et al [21] found only 77% and 93% abnormalities respectively.
Conclusion
The present study concluded that most common cause of pancytopenia is aplastic anaemia, followed by megaloblastic anaemia and acute leukemia. BMA coupled with BMB is helpful for understanding disease process and to find out the cause in the majority. BMB is the diagnostic in dry tap cases, is also useful in detecting granuloma and in staging of lymphoma. Of course the role of immunophenotyping, IHC, cytogenetics and molecular diagnosis can’t be ignored carrying many diagnostic and prognostic information. However, in a resource poor setting a proper history, clinical examination, good haematological work up along with BMA and trephine biopsy will be enough for evaluation of pancytopenia.
Conflict of Interest
The authors declare that they have no conflict of interest.
REFERENCES
- Rao KS. Pancytopenia: a clinico hematological study. J Lab Physicians. 2011;3:15-20.
- Khodke K, Marwah S, Buxi G, Yadav RB, Chaturvedi NK. Bone Marrow Examination in Cases of Pancytopenia. J Ind Acad Clin Med. 2001;2:55-59.
- Shah P, Patel RD, Gamit B, Gheewala S. Bone marrow examination in cases of pancytopenia. Int J Res Med Sci. 2017;5:1494-1498.
- Tilak V, Jain R. Pancytopenia--A clinico-hematologic analysis of 77 cases. Indian J Pathol Microbiol. 1999;42:399-404.
- Camitta BM, Thomas ED, Nathan DG, Santos G, Gordon-Smith EC, Gale RP, et al. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood. 1976; 48:63-70.
- Bacigalupo A, Hows J, Gluckman E, Nissen C, Marsh J, Van Lint MT, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT* SAA Working Party. Br J Haematol. 1988;70:177-182.
- Varma N, Dash S. A reappraisal of underlying pathology in adult patients presenting with pancytopenia. Trop Geogr Med. 1992;44:322.
- Kumar R, Kalra SP, Kumar H, Anand AC, Madan H. Pancytopenia--a six year study. J Assoc Phys India. 2001;49:1078-1081.
- Khunger JM, Arulselvi S, Sharma U, Ranga S, Talib VH. Pancytopenia--a clinico haematological study of 200 cases. Indian J Pathol Microbiol. 2002;45:375-379.
- Biswajit H, Pratim PP, Kumar ST, Shilpi S, Krishna GB, Aditi A. Aplastic anemia: a common hematological abnormality among peripheral pancytopenia. N Am J Med Sci. 2012;4:384.
- Jyoti SK, Badhe BA, Dutta TK, Sajjan J. Clinicopathological Study of Adult Pancytopenia with Special Reference to Bone Marrow Biopsy. Int J Blood Dis Dis. 2019;3:8-13.
- Aziz T, Ali L, Ansari T, Liaquat HB, Shah S, Ara J. Pancytopenia: megaloblastic anemia is still the commonest cause. Pak J Med Sci. 2010;26:132-136.
- Dasgupta S, Mandal PK, Chakrabarti S. Etiology of Pancytopenia: An observation from a referral medical institution of Eastern Region of India. J Lab Physicians. 2015;7:90-95.
- Devi PM, Laishram RS, Sharma PS, Singh AM, Singh MK, Singh YM. Clinico-hematological profile of pancytopenia in Manipur, India. Kuwait med J. 2008;40:221-324.
- Jain A, Naniwadekar M. An etiological reappraisal of pancytopenia-largest series reported to date from a single tertiary care teaching hospital. BMC Blood Disorders. 2013;13:10.
- Toi PC, Varghese RG, Rai R. Comparative evaluation of simultaneous bone marrow aspiration and bone marrow biopsy: an institutional experience. Indian J Hematol Blood Transfus. 2010;26:41-44.
- Hunt BJ, Andrews V, Pettingale KW. The significance of pancytopenia in miliary tuberculosis. Postgrad. Med. J. 1987;63:801-804.
- Navone R, Colombano MT. Histopathological trephine biopsy findings in cases of dry tap bone marrow aspirations. Appl Pathol. 1984;2:264-271.
- Pandya A, Patel T, Shah N. Comparative utility of bone marrow aspiration and bone marrow biopsy. J Evol Med Dent Sci. 2012;1:987-993.
- Engeset A, Nesheim A, Sokolowski J. Incidence of ‘dry tap’on bone marrow aspirations in lymphomas and carcinomas: diagnostic value of the small material in the needle. Scand J Haematol. 1979;22:417-422.
- Humphries JE. Dry tap bone marrow aspiration: clinical significance. Am J Hematol 1990;35:247-50.
Citation: Mohanty P, Swain R, Rani SS, Sethy S (2020) Bone Marrow Biopsy Evaluation in Cases of Pancytopenia-An Institutional Experience. J Blood Disord Transfus 11: 435
Copyright: © 2020 Mohanty P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited

