Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review - (2020) Volume 12, Issue 5
Biologics and Biosimilars - A Review on their Therapeutics Activities
Chetan Suthar*Received: 14-Aug-2020 Published: 27-Oct-2020, DOI: 10.35248/0975-0851.20.12.401
Abstract
Biologics medicine has a great advance in the treatment of serious illness. The manufacturing of these molecules which are large and complex is quite difficult because they are been made in the living cells grown in the laboratory. The replica of biologic medicine is impossible for the manufacturer to manufacture due to some of the factors such as the inherent complexity of the biologics and the proprietary details of the manufacturing process for the original biological medicine often can be referred to as the reference products. Due to this, the copies of the biological products produced are called as biosimilar, the biosimilar is highly similar to biological products but not identical.
Keywords
Biologics medicine; Biological products; Pharmacist; Biosimilars
Introduction
In the United States and other countries biological products are rapidly growing products with therapeutics activities. The cost of the biological products is higher as compare to the biosimilar and interchangeable products which can be also been prescribed to the patient to lower the health care cost [1-3].
In various countries like U.S., Europe, Japan, Australia, and Canada regulatory authorities require a developer to get a highquality standard to demonstrate the similarity between the biosimilar products and the reference products. The manufacturer of the biosimilar data should have to provide a robust data to demonstrate that there is no clinical difference is existing between the biosimilar and the original medicine [4-8].
There are several clinical considerations for biosimilar they are:
• The biosimilar label should contain all the relevant information regards the safety and use of biosimilar so that an informed choice can be made regard the appropriate medicine for the patient.
• The biologics and biosimilar should have different names for better understanding. All the members in the patient care should have a clear understanding of the medicine like by which manufacturer is manufactured and being administered to the patient.
• Substitution is a practice performed by the pharmacist in which he may dispense an alternative medicine for the medicine which is prescribed by the physician. In the U.S. this is done for the biosimilar which has achieved an interchangeability which is given by the FDA and this legislation had been approved in some of the states for the substitution of biosimilars. In countries like Europe, there is no substitution of the biosimilar is been practiced by the pharmacist and several countries also implemented laws against this [9-11].
Literature Review
Biosimilar plays an important role in healthcare providing patients and the physician more therapeutic options. Serving the patients is what we do and this is the reason why we are leveraging the scientific capabilities from more than three decades of the experience in the biologics for developing biosimilar with high qualities.
Biologics products
According to USFDA biological products are large complex molecules. These products are produced through biotechnology in a living system such as plant cell/animal cell and micro-organism and are more difficult to characterize than the small molecules. Biological products re been regulated by the FDA and are used for the prevention, diagnosis, treat and cure of the diseases, illness or medical conditions. There is a large range of biological products that been used in the U.S. including the monoclonal antibodies (adalimumab), therapeutics proteins (filgrastim) and some of the vaccines (for tetanus & influenza) [12].
According to Amgen biological medicine are those that are large and complex and grown in a laboratory by living cell. The size of the Biological molecules is 200 to 1000 times the size of the small molecules or the chemical drug such as aspirin [13-16]. Biological medicines are injected into the body due to their large molecular size and fragile structure [2,3,17,18].
Biosimilars
According to USFDA biosimilar is the products that are mostly similar to the biological products and does not have any clinically different from the existing FDA approved reference products [12] (Figure 1).
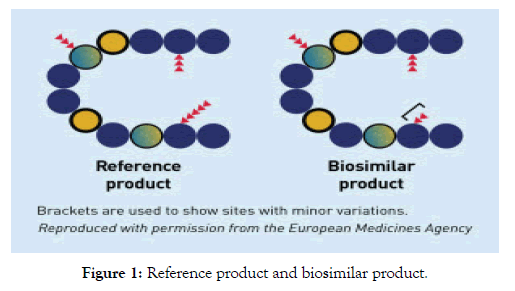
Figure 1: Reference product and biosimilar product.
According to Amgen biosimilar is the biological medicine that is similar but not identically as that of the original biological medicine [1]. The biosimilar cannot be possible to have the exact copy of the biological products because the biologics are developed using cell lines that are unique to a given manufacturer and are made by using various purification processes. Therefore the copies are highly similar but bot identical to the original biologic upon which they are based.
According to Pfizer biosimilar product is the biologic products that are approved based in that it is highly similar an approved biological products by FDA known as the reference product and which do not have any clinical difference in the safety and effectiveness as compared to the reference product [14]. Only some of the minor differences in the clinically inactive components are allowable in the biosimilar products.
Reference products
Reference products are the single biological products that had been already approved by the FDA, against which a biosimilar product is compared. Reference products are approved on the basis of safety and effectiveness. Biosimilar products are compared and evaluated against the reference products to find that the product should not have any clinical differences and the products should be highly similar [12].
Highly similar
Developing proposed biosimilar demonstrates by the manufacturer whose product is similar to the reference product by analyzing its function and structure of both the proposed biosimilar and the reference product. For demonstrating that the biosimilar is highly similar to that of the reference product the manufacturer uses the result which is taken from the comparative test such as purity, chemical identity, and bioactivity. Some of the minor differences in the clinically inactive component between the reference product and the proposed biosimilar product are acceptable [19]. For example, there may be a difference in the buffer or the stabilizer compared to which been used in the reference product. The difference in the proposed biosimilar products and the reference product are evaluated by the FDA so that the biosimilar can meet the FDA high approval standards.
For the biosimilar and the reference product lot-to-lot difference (i.e., acceptable within product difference) are been carefully monitored and controlled and for the biological product, there is slightly (i.e., acceptable within-product variation) difference expected during the manufacturing process.
By demonstrating the human pharmacodynamics and pharmacokinetic studies the manufacturer also has to prove that the proposed biosimilar product has no clinical difference as compared to the reference products in case of safety and effectiveness [12].
Interchangeable products
Interchangeable products are the biological products that should meet the additional requirement given by the Biologics Price Competition and Innovation Act. The information is also needed to show that the interchangeable product has the same clinical results as that of the reference products in any of the given patient [12].
Without the involvement of the prescriber, interchangeable products can be substituted in place of the reference products. The FDA high standard for approval assures to the health care provider that they can be confident in terms of safety and efficacy of the interchangeable product (Figures 2 and 3).
Figure 2: Characterization of the biosimilar and biological products.
Figure 3: Biosimilar development.
Biosimilar in the treatment of cancer
The ability of the biologics to target directly to specific cancer cells makes them important for use in cancer treatment. In treatment to fight against cancer many biologics work by using the immune system of the patient, the biologic doesn't kill more healthy tissue as compared with the other drug treatment and they have fewer side effects and less toxicity [16].
The biologics i.e., the antibodies are used in the destruction of the cancer cell but some of them work as supportive by reducing the side effects of the chemotherapy or the radiation treatment.
Example- Colony-stimulating factor (CSFs) class of biologics they encourage one marrow to grow and to divide; besides that CSFs also increase the number of white blood cells in the body which help the patient to tolerate the chemotherapy at higher doses.
Manufacturing of the biological products
Biological medicines are manufactured by using the living cell via a complex multi-step process they are given below (Figure 4).
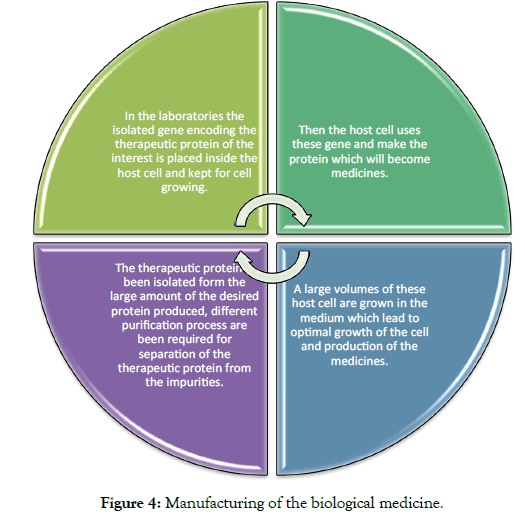
Figure 4: Manufacturing of the biological medicine.
Manufacturing of the biosimilar products
Some of the steps for the manufacturing of the biosimilars are: and are explained below (Figure 5).
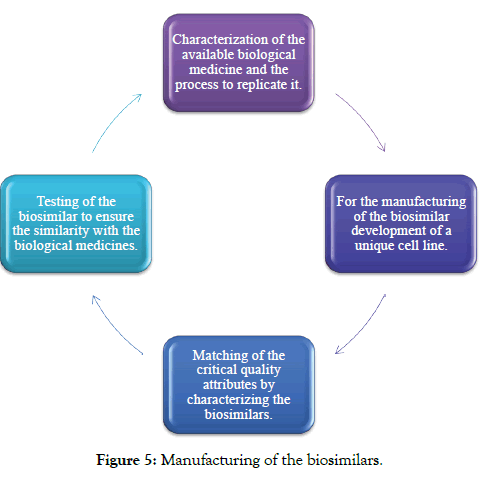
Figure 5: Manufacturing of the biosimilars.
Developing and testing biosimilars
Step-1: For developing of the biosimilar there is the requirement of scientific and manufacturing expertise due to the structural complexity of the biological medicines. For developing a biosimilar the manufacturer show know the biological medicine working in the body and understanding of the biological medicines.
The developer should work on developing its process for the production of the biosimilar and the developer should not have the proprietary knowledge of the manufacturing process of the biological medicines [1].
Step-2: The biological and the biosimilar products are produced in the living cell in a multi-step process and that is different or unique for every manufacturer, the detail of the manufacturing will be slightly different between the biosimilar and the original biological products, due to this there will be a minor change in the final products. The slight difference in the manufacturing detail may affect its quality, safety and the effectiveness of the products. It is critical to find the difference and to demonstrate that they are not clinically meaningful, but the biosimilar is expected to be safe and effective as compared to the original biological medicine.
Step-3: The biological drug is very complex and has a large number of features. In biological there are a hundred or more attributes. Some of the attributes are important in many ways the body can recognize the present protein and it may be critical to the safety and efficacy and some of pharmacokinetic of the drug. The features which are important of these functions are also called as critical quality attributes (CQA). This CQA includes many structural aspects of the molecules that are influenced by the cell line and the DNA and the manufacturing process [20]. Critical quality attributes may impact on clinical activity that is the way molecule affect the patient, the CQA should fall within the expected range given. The manufacturer of the biosimilar uses more than 40 analytical tests to determine 100 of attributes [21,22].
Step-4: Many of the tests are such as preclinical assays, clinical evaluations are used to show that the biosimilar has no meaningful difference from the original biological medicine, all the information from the clinical, nonclinical and analytical are integrated to provide an assessment call as the totality of the evidence, some of the below are the contributes in the totality of evidence are as follows [23]:
1. Analytical characterization: To show the biosimilar matched all the critical quality attributes and function of the original biological products different testing are done in the laboratory. It for the foundation of the bio similarity assessment.
2. Nonclinical testing: Different studies on the animals are done to assess the activity, function, and toxicity of biosimilar.
3. Final step in demonstrating bio-similarity:
► Clinical studies in humans- In clinical human studies, two phases of the clinical studies are required:
► Phase-1- Studies are done to demonstrate the pharmacokinetic and pharmacodynamics.
► Phase-3-Studies are done to demonstrate similar efficacy, immunogenicity, and safety to the reference biological product [24].
After the demonstration, the pharmacokinetic and pharmacodynamics equivalence the biosimilar is developed to conduct a pivotal clinical trial. The main importance of the pivotal clinical trial is to prove that there is no clinically meaningful difference between the proposed and the original biological medicine. This also means that the proposed biosimilar product is neither inferior nor superior to the original biological medicine. For the clinical trial for the biosimilar, the population selected should react to all the clinically meaningful differences between the original biological and biosimilar and this type of the population is known as the most sensitive population. The study length should be done properly to allow the detection of any immune response, which sometimes lags in the receipt of the medicine [4] (Table 1 and Figure 6).
Table 1: Main difference between the generic biologics and biosimilar [17].
| Variables | Generics | Biosimilars | Biologics |
|---|---|---|---|
| Development cost (USD) | 2-3 millions | 800 million | 100-300 million |
| Time to market (years) | 2-3 millions | 10-Aug | 8-Jul |
| Clinical studies | Bioequivalence studies in healthy volunteers | Phase 1-3 studies efficacy and safety | Pharmacokinetic comparison studies in Phase 3 |
| Patients | 20-50 | 800-1000 | ~100-500 |
| Post-authorization activities | Pharmacovigilance | Phase 4, risk management plan including pharmacovigilance | Phase 4, risk management plan including pharmacovigilance |
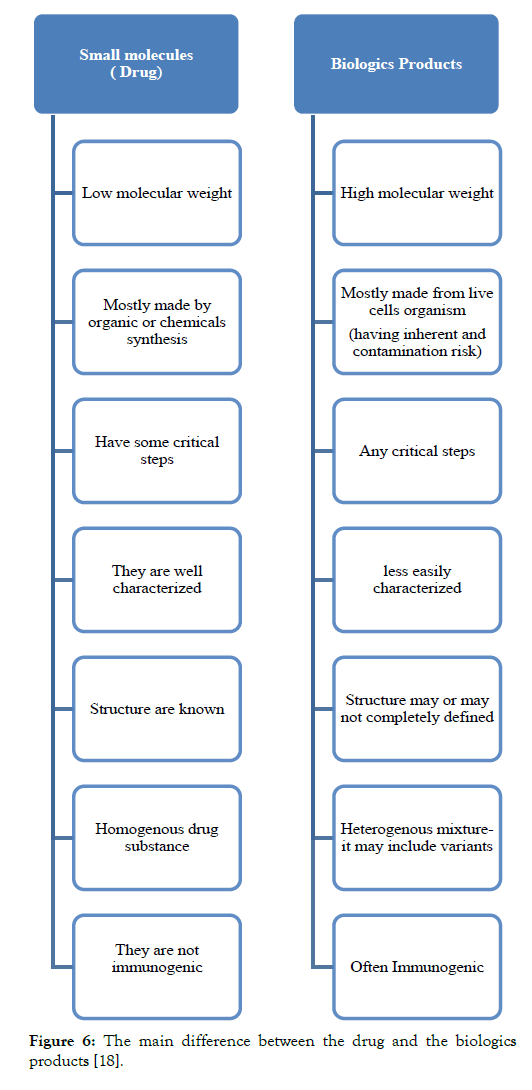
Figure 6: The main difference between the drug and the biologics products [18].
Abbreviated license pathway for biological products
Biological products that are similar to an FDA -licensed reference product may rely on license on and among some of the other things like publicly available and some of the information regarding the FDA previously determined that the biological products ( reference products) are safe more potent and pure.
The biosimilar products are licensed under the 351 (k) of the Public Health Service Act based on the preclinical and clinical data - abbreviated license pathway.
General requirements
Application 351 (k) including the information that demonstrates that the biological products is-
1. The same mechanism of action for the required condition of use.
2. It is biosimilar to a reference product.
3. For the reference products, the condition of use in labeling had been previously approved.
4. It should have the same dosage form, strength, and route of administration as the reference products.
General requirements 351 (k) application
The Public service act requires that 351 (k) applications should include the other things and the information demonstrating that bio similarity based upon the data derived from:
Analytical studies: It demonstrates that the biological products are highly similar to that of the reference products without any minor difference in the inactive components.
Animal studies-including the toxicity
Clinical studies: The information that is required for the safety purity and potency in 1 or more condition of the use for which the reference products has been licensed, and for which grating of the license for biological products.
Standards for granting of license
FDA shall grant the license for the biological products under section 351 (k) of PHS act if FDA checks that the information which has been submitted by the applicant is sufficient enough to show that the:
• Biological products ID biosimilar to the reference Product.
• It should meet all the standards that are given in 351 (k) (4) and therefore it is interchangeable with the reference products.
• Permission of the applicant for the inspection of the facility under section 351 (c).
Non-U.S.-licensed comparator products
According to the PHS act for 351 (k) the "reference products" are defined as the "single biological products which are licensed under the 351 (k) against which the biological products are evaluated".
The data from the animals and the clinical studies comparing with the non-US-licensed biosimilar product may be used to support the bio-similarity to a U.S.-licensed reference product. The information and the date should be provided by the sponsor to scientifically justify the quality of these comparative data to the assessment of the bio-similarity and to establish an acceptable bridge to the U.S. licensed reference product.
Support for the use of the non-us-licensed comparator
Type of the bridging data that needed would include the following:
• Comparison of physicochemical (directly) of the total 3 products (Proposed biosimilar to non-US-licensed comparator products; proposed biosimilar to US-licensed reference product; US-licensed reference products to non- US-licensed comparator product)
• All the bridging of the 3 clinical PK and PD studies.
• The acceptance criteria should meet all the pre-specified acceptance criteria for the PK and PD similarity of all the three pairs.
Regulating of biosimilars
The approaches which are established for the generic drug are not suitable for the evaluation, development and licensing of similar biotherapeutics products, because the biotherapeutics products consist of complex and large protein which are difficult to characterize.
World Health Organization (WHO)
For the evaluation and approval of the biosimilar WHO released the guidelines in 2009 with globally accepting principles [20]. Many of the other countries had used these guidelines to generate or to make their country-specific guidelines.
Europe and the European Medicine Agency (EMA)
The European guidelines were adopted in 2005 and updated in 2015. It was the first country to develop biosimilar guidelines in response to emerging patient deadlines for the number of biological medicines [21].
The European guidelines were accepted by Australia and they adopted them in the entire country. EMA has issued guidelines which are specific to the clinical and non-clinical requirements, quality manufacturing, manufacturing changes, and immunogenicity, they also released some of the guidelines that are for describing the requirement for the bio-similarity demonstration of specific medicines [5,22-25].
North America: U.S. AND FDA
FDA in February 2012 had issued 3 guidance documents for the biosimilar products development for assisting those companies that are developing biosimilars in the U.S. In the year 2015 April FDA revised updated and finalized these guidelines and they are as follows:
• Quality consideration in demonstrating bio-similarity of therapeutic protein products to reference products [19].
• Biosimilars: Questions and answers regarding implementation of the biologics price competition and innovation act of 2009 guidance for industry [11].
• Scientific consideration in demonstrating bio-similarity to a reference product [4].
In addition to the above 3 guidelines, FDA has issued many different guidelines regarding labeling, names, clinical, pharmacology, reference products, demonstrating interchangeability with a reference product, and how to approach formal meetings with FDA [26-31] (Figure 7).
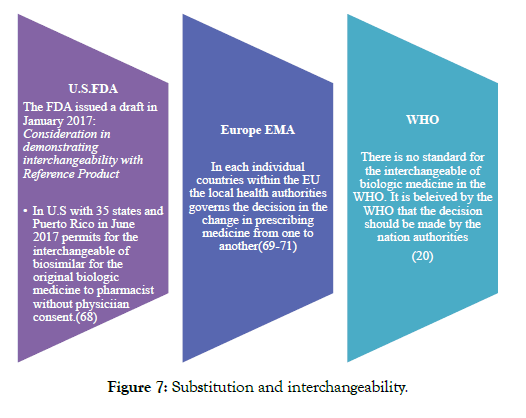
Figure 7: Substitution and interchangeability.
Canada and Health Canada
Health Canada adopted its guidelines in 2010 and releases its updates of the biosimilar guidelines in 2016 [7]. For the additional information, Healthy Canada has released a question and answer document along with the guidelines [32].
Latin American region
In Argentina, the biosimilar is also known as the Medicamento biológico similar. The biosimilar guidelines for the Argentine were released in the year 2008 by the ANMAT. The Guidelines which were released by the ANMAT follows the principal of the European Biosimilar Guidance [33].
Brazil and Anvisa
Brazil had developed its guidelines for the biosimilar regulation in 2010 (Resolution no. 55/2010) and the biosimilar products in Brazil are known as the follow-on biological products. The Brazil guidelines follow the principle of WHO bio-similar guidance [34].
Colombia and Invima
The final guidelines in Colombia for the biosimilar products were released in September 2014. The Biosimilar products in Colombia are also known as the productos bioterapéuticos similares [35].
Cuba and CECMED
CECMED published their biosimilar guidelines in the year 2011. In Cuba, the biosimilar is also known as the Biosimilar Products. The Guidelines which are published by the CECMED are based on WHO guidelines with some of the adjustments to ensure that they are appropriate for the regions [36].
The official decree provided in the guidelines for three routes for biological products are: Comparability route, Complete Route, Abbreviated route, which aims for the approval of the biosimilars. Some of countries like the U.S. and E.U. had raised some concerns with the decree of the Colombia likely to put patient safety at risk and not having the sufficient details.
Mexico and Cofepris
In Mexico the biosimilar guidelines came into effect in April 2012. The biosimilar products are also known as the bio-comparable [37]. In the market of Mexican 23 non-originator biologics are available. Prior to the October 2011 the companies who registered to the biologics are now mandate to prove the bio-similarity by conducting the clinical trials. In 2012 the biosimilar guidelines had also been issued in Costa Rica in 2012, In Gautemala 2010, Peru 2011, Uruguay 2015 and in Venezuela 2012 [38-42].
Australia and the Therapeutics Good Administration (TGA)
The EMA Biosimilar guidelines in the Australia were adopted in the year 2008 [8] (Figure 8).
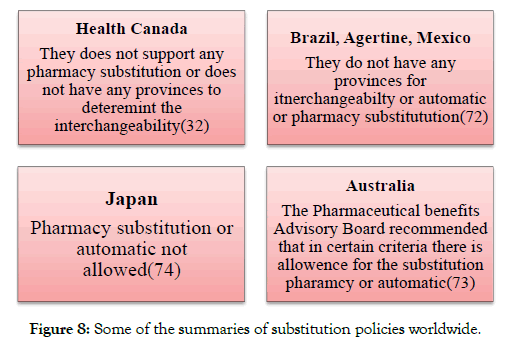
Figure 8: Some of the summaries of substitution policies worldwide.
Asia and Asia Pacific
The Biosimilar in the Japan are also known as the Follow on Biologics (FOBs). The guidelines in the Japan are developed in 2009. The guidelines of the Japan are based on the principle of EU biosimilar guideline. In addition the PMDA has also issued many different guidelines such as naming, marketing approval applications, and some of the extra documents containing the questions and answers [6].
Korean Federal Drug Association (KFDA)
In 2010 the guideline on the evaluation of the biosimilar products came into existence and this guidelines was updated in 2014. The guidelines of the Korea are based on the Japanese, Europe and WHO guidelines. For demonstrating bio-similarity for some of the specific medicines KFDA had issued a set of the guidelines [43] (Figure 9).
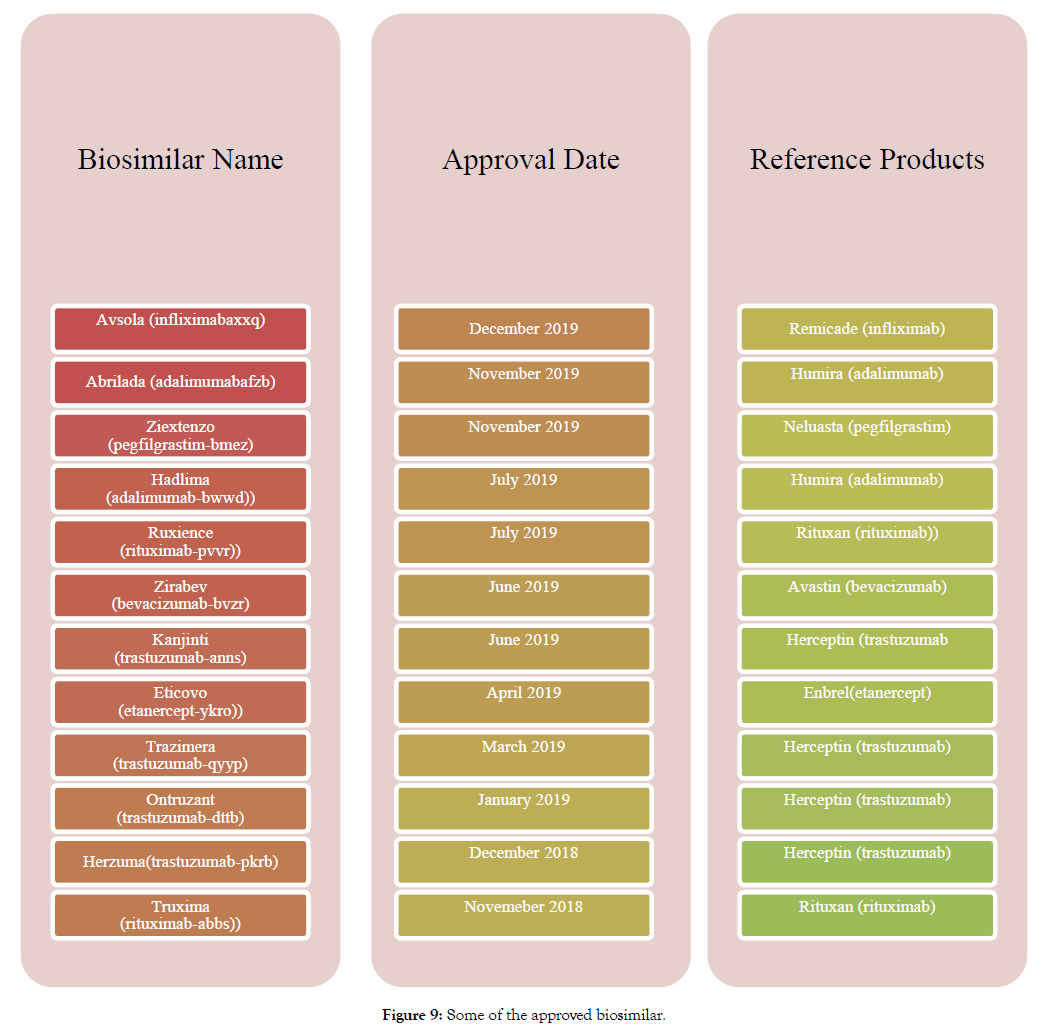
Figure 9: Some of the approved biosimilar.
Taiwan and Taiwan FDA (TFDA)
In 2008 TFDA issued their guidelines that are guidance for review and approval of biosimilars. They also published two additional guidelines that are:
• In 2010- Points to consider for review and approval of biosimilar products.
• In 2013- Guidelines for review and approval of biosimilar monoclonal antibodies [44].
Biosimilar guidelines have been issued in many different countries in difference year and they are as follows: [45-60] (Tables 1 and 2). In 2012 India had released official guidelines and before the introducing the guidelines about 20 biosimilar had approved for used and they are under the hoc abbreviated process. The progress is monitored by the WHO [60].
Table 2: Biosimilar guidelines of different countries.
| Countries | Year |
|---|---|
| Malaysia | 2008 |
| Singapore | 2009 and updated in 2012 |
| China | 2015 |
| Iran | 2010 |
| Israel | 2009 |
| Jordan | 2013 |
| Kazakhstan | 2009 |
| Philippines | 2014 |
| Saudi Arabia | 2010 |
| Thailand | 2012 |
| Egypt | 2012 |
| Moldova | 2012 |
| Nigeria | 2012 |
| South Africa | 2012 |
| Turkey | 2012 |
| Ukraine | 2013 |
Biosimilar approved around the world
In 2017 about eight classes of biosimilar medicines are approved around the world not all are been approved in all the region bus some variation in the specific diseases for which the medicines has been approved [61-67].
Clinical considerations
Clinical consideration is that when and how the biological and the biosimilar can be safely used. There can be switch in the biologic medicine from which a patient is currently been treated with another biologic either by the biosimilar or by different brands by the clinician and they should be available at the same condition for treating [62]. This decision is made for consulting the patient by the health provider. Substitution is a practice done by the pharmacist in which he can dispense any alternative medicine for the prescribed medicine without any approval from the physician. In the U.S. this is done for the biosimilar which has been designated by the FDA named as “interchangeable” and it is applicable in only those countries in which legislation approved or the regulation is established for the biosimilar substitution [68-75]. In countries like Europe there are no such regulation and the pharmacist don’t have the right to substitute the biosimilar products by any other similar products.
Conclusion
The biosimilar which been administered have distinguishable names so the healthcare professional and the patient can easily understand the biosimilar. For the prescribing physician it is important that the labels should contain the information regard the safety and data behind the approval so informed choice can be made for appropriate medicine for the patient.
REFERENCES
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19:411-419.
- Roger SD. Biosimilars: How similar or dissimilar are they? Nephrology. 2006;11:341 346.
- Gottlieb S. Biosimilars: Policy, clinical, and regulatory considerations. Am J Health Syst Pharm. 2008;65(14 suppl 6):S2-S8.
- U.S. Food and Drug Administration. Guidance for industry: Scientific considerations in demonstrating biosimilarity to a reference product. 2015.
- European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. 2006.
- Guideline for the Quality, Safety, and Efficacy Assurance of Followon Biologics. Japan. 2009.
- Health Canada. Guidance for Sponsors: Information and Submission Requirements for Subsequent Entry Biologics (SEBs). 2016.
- The Therapeutic Goods Administration, Australia. https://www.tga.gov.au/
- European Commission. What you need to know about biosimilar medicinal products: A consensus information paper. 2013.
- Olech E. Biosimilars: Rationale and current regulatory landscape. Semin Arthritis Rheum. 2016;45:S1-S10.
- U.S. Food and Drug Administration. Guidance for industry: Biosimilars: Questions and answers regarding implementation of the Biologics Price Competition and Innovation Act of 2009. 2015.
- https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products.
- Morrow T, Felcone LH. Defining the difference: What makes biologics unique. Biotechnol Healthc. 2004;1:24-29.
- https://www.pfizerbiosimilars.com/
- https://www.kymos.com/content/characterization-biologics-and-biosimilars
- https://www.fightcancer.org/policy-resources/understanding-biologic-and-biosimilar-drugs
- Rodríguez de la Cuerda AL. Biosim. Biosimilares en España. Presentación XI Encuentro de Autoridades Competentes en Medicamentos de los Países Iberoamericanos (EAMI) “De la estrategia a la acción en los medicamentos”. 22nd June 2016. Varadero, Cuba.
- www.fda.gov
- U.S. Food and Drug Administration. Guidance for industry: Quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference protein product. 2016.
- World Health Organization Expert Committee on Biological Standardization. Guidelines on evaluation of Similar Biotherapeutic Products (SBPs). 2009.
- European Medicines Agency. Guideline on similar biological medicinal products. 2005.
- European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues (revision 1). 2014.
- European Medicines Agency. Guideline on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical use. 2012.
- European Medicines Agency. Guideline on immunogenicity assessment of biotechnology-derived therapeutic proteins. 2016.
- European Medicines Agency. Guideline on comparability of biotechnologyderived medicinal products after a change in the manufacturing process - non-clinical and clinical issues. 2007.
- U.S. Food and Drug Administration. Guidance for Industry: Nonproprietary naming of biological products. 2017.
- U.S. Food and Drug Administration. Guidance for Industry: Considerations in demonstrating interchangeability with a reference product. Draft guidance. 2017.
- U.S. Food and Drug Administration. Guidance for Industry: Labeling for biosimilar products. 2016.
- U.S. Food and Drug Administration. Guidance for industry: Clinical pharmacology data to support a demonstration of biosimilarity to a reference product. Draft guidance. 2014.
- U.S. Food and Drug Administration. Guidance for industry: Reference product exclusivity for biological products filed under Section 351(a) of the PHS Act. Draft Guidance. 2014.
- U.S. Food and Drug Administration. Guidance for industry: Formal meetings between the FDA and biosimilar biological product sponsors or applicants. 2015.
- Health Canada. Fact Sheet: Biosimilars.
- Argentina. Administración Nacional de Medicamentos, Alimentos y Tecnología Médica (ANMAT) Disposition Number 7729. 2016.
- Brazil. National Health Surveillance Agency Collegiate Board. Resolution RDC No. 55, 2010.
- Colombia. Instituto Nacional de Vigilancia de Medicamentos Y Alimentos (INVIMA). Pharmacological Evaluation of Biological Drugs. 2014.
- Cuba. Centro Para el Control Estatal de la Calidad de los Medicamentos (CECMED). Regulation No. 56: Requirements for Marketing Authorization of Known Biological Products. 2011.
- Mexico. Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS). Norma Oficial Mexicana NOM-257-SSA1-2014, En Materia de Medicamentos Biotecnologicos.
- Costa Rica. Regulation of Similar Biotherapeutic Products in Latin America. 2013.
- Guatemala. Ministerio de Salud Pública y Asistencia Social. Registro Sanitario de Referencia de Productos Biologicos y Biotecnologicos Technical standard 67-2010.
- Peru. Normas Legales 447499. Capitulo V. De los Productos Biologicos.
- Uruguay. Registro de Medicamentos Biotechnologics. 2015.
- Venezuela. Norma para Registro Sanitario y Farmacovigilancia de Productos Bioterapeuticos Similares en la Republica Bolivariana de Venezuela. 2012.
- Korea. Guidelines on the Evaluation of Biosimilar Products.
- Taiwan. Guideline on the Examination and Registration of Drugs – the Guideline on Biosimilar Products.
- Malaysia. Guidance Document and Guidelines for Registration of Biosimilars in in Malaysia.
- Singapore. Guidance on Registration of Similar Biological Products in Singapore.
- China. Ropes and Gray. China Announces Final Biosimilars Guideline. 2015.
- Iran. Guideline for Biosimilar Products.
- Israel. A New Policy Regarding the Registration and Use of Biosimilar Pharmaceuticals in Israel. 2014.
- Jordan Food & Drug Administration. Guidance for Registration of Biosimilars. 2013.
- Kazakhstan. The Approaches to the Regulation of Biological Medicinal Products in the Republic of Kazakhstan. 2009.
- Philippines Food and Drug Administration. Guidelines on Evaluation of Similar Biotherapeutic Products (SBPs) for the Registration of Biosimilar Products. 2014.
- Saudi Arabia. Guidelines on Biosimilars. Version 2.1. 2010.
- Thailand. (Draft) Guidelines for Regulating Biosimilars in Thailand, Circular, 2012.
- Egypt. Draft Guideline for the Registration of Biosimilar Products. 2012.
- Moldova. Order No. 739 of the Ministry of Health: Regulation on the Marketing Authorization of Medicinal Products. 2012.
- Nigeria. Guidelines for the Registration of Biosimilars in Nigeria. 2012.
- South Africa. Biosimilar Medicines – Quality, Non-clinical, and Clinical Requirements. 2012.
- Turkey. Turkish Guidelines for Biosimilars. 2012.
- Ukraine. Ministry of Health of Ukraine: Orders Pertinant to Registration of Medicinal Products. 2013.
- India. Guidelines on Similar Biologics: Regulatory Requirements for Marketing Authorization in India. 2012.
- RAPS. Regulatory Explainer: Everything You Need to Know About Biosimilars. August 10, 2016.
- The Korea Herald. Celltrion’s Rituxan biosimilar receives sales approval in Korea. 2016.
- Quintiles IMS. Approved biosimilars in Europe.
- GaBI Online. Biosimilars approved in Australia. 2017.
- GaBI Online. Biosimilars approved in Japan. 2016.
- Quintiles IMS. Approved SEBs in Canada.
- Food and Drug Administration. FDA approves first biosimilar product Zarxio. 2015.
- National Conference of State Legislatures. State Laws and Legislation Related to Biologic Medication and Substitution of Biosimilars.
- GaBI Online. Benepali wins Danish tender for etanercept. 2016.
- GaBI Online. Biosimilar infliximab offered to French hospitals at 45% discount. 2015.
- European Commission. Public Procurement Directive 2014/24/EC. 2016.
- Garcia R, Araujo DV. The regulation of biosimilars in Latin America. Curr Rheumatol Rep. 2016;18:16.
- GaBI Online. Australia’s PBAC recommends substitution of biosimilars. 2015.
- Nagai S, Yanagihara R, Kishioka Y. Japanese regulatory authority’s perspective on biosimilars. Lancet. 2015;16:e101.
Citation: Suthar C (2020) Biologics and Biosimilars - A Review on their Therapeutics Activities. J Bioequiv Availab 12:401. doi: 10.35248/0975-0851.20.12.401.
Copyright: © 2020 Suthar C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

