Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 12
Biofumigation Potentials of Some Brassica Crops to Control Root-knot Nematodes Meloidogyne spp., on Tomato Under Field Conditions
Shimaa Hassan1, Amal A Al-Gendy2, Sahar H Abdel-Baset3*, Salah M Abd El-Kareem1, Samia Massoud4 and Mohamed Abdallah52Department of Pharmacognosy, Faculty of Pharmacy, Egypt
3Department of Nematology, Plant Pathology Res. Inst., ARC, Giza, Egypt
4Department of Agricultural Botany, Faculty of Agriculture, Suez Canal University, Egypt
5Faculty of Desert and Environmental Agriculture,, Matrouh University, Egypt
Received: 07-Nov-2020 Published: 27-Dec-2020, DOI: 10.35248/2157-7471.20.12.227
Abstract
The experiments were conducted to study the efficacy of fodder radish (Raphanus sativus var. Terranovah) and rocket salad (Eruca sativa cv. Baladi) as biofumigation crops for controlling root-knot nematodes Meloidogyne spp., on tomato plants during two successive seasons 2017 and 2018 under field conditions. Three months after cultivation of fodder radish, and rocket salad (the full blooming stage) all parts were incorporated with soil and covered with a transparent polyethylene film. After 4 weeks, the plastic sheets were removed and soil was left for two weeks later, before transplanting tomato seedlings. Results in both seasons, indicated significant reduction (p≤0.05) of nematode parameters on tomato plants. Results revealed that effect of R. sativus var. Terranovah as biofumigation crop as indicated by the percentage reduction in number of galls, egg-masses/root system, and a number of second-stage juvenile (j2) /250 g soil (84, 90, and 84%), and (90,87, and 88%) in seasons 2017 & 2018 respectively. On the other hand, nematicide Vydate (oxamyl) 24% L recorded the percentage reduction in the number of galls, egg-mass/ root system as well as number of second-stage juveniles in soils j2 (90,87, and 87%), and (95,93, and 90%) in season 2017& 2018 respectively. Results revealed that all plant growth criteria on tomato plants increased significantly (p≤0.05) by using the tested treatments. Results indicated that the effect of R. sativus as biofumigation crop recorded the average highest percentages (57, and 92%), and (64, and 102%) in seasons 2017 &2018 respectively. At the same time, nematicide Vydate (oxamyl) 24% L was the most effective in the average highest percentages. i.e., the plant growth vigor, increase, and fruit yield per plant (64, and 98), (73, and 107) in two successive seasons 2017 & 2018 respectively.
Gas liquid chromatography-mass spectrometry (GLC-MS) analysis of dichloromethane extract of fodder radish indicated the presence of four glucosinolates, which were identified through their volatile autolysis products. Gluconapin, the major compound was identified by 3- butenyl isothiocynate, while glucoerucin was identified by 4-(methylthio) butyl isothiocynate, which is commonly known as erucin. Sulphoraphane was released from 4-(methylsulfinyl) butyl glucosinolate (glucoraphanin) while 4-(methylsulphony) butyl isothiocyanate commonly known as erysolin was liberated from glucoerysolin. Furthermore, five GLS were also identified in rocket salad. Gluconapin was detected and its presence was identified through its epithionitrile; 4,5-epithiopentanenitrile. The isomers progoitrin and epiprogoitrin were detected by the two hydrolysis products diastereomers threo and erythro 1-cyano-2-hydroxy-3,4-epithiobutane respectively. The aromatic glucosinolate; gluconasturtiin could be identified through its liberated nitrile named as 1-benzenepropane nitrile. Sativin, the major identified compound is 4-mercaptobutyl isothiocyanate.
Keywords
Meloidogyne spp; tomato (Solanum lycopersicum L.); biofumigation; Raphanus sativus; Eruca sativa; Isothiocyanates; Glucosinolates.
Introduction
Tomato (Solanum lycopersicum L.) is the second most important vegetable crops in the world next to potato with approximately 182.3 million tons of tomato fruits produced on 4.85 million ha each year, Europe, America, and Africa produced 13.5, 13.4, and 11.8% of the total tomato yield, respectively [1]. Egypt is the fifth country in the world in terms of the production of tomatoes. It's grown all year round. The cultivated area reached 475.514 thousand feddan produced approximately 7.94 million tons [2].
Among the several pests and diseases that affect tomato plants, the plant-parasitic nematodes pose a major threat, resulting in an estimated annual monitoring loss of USD 80 billion [3]. The most prevalent and destructive among the phytonematodes of tomato are Meloidogyne spp., which causes major economic losses on vegetables, especially in tropical and subtropical areas [4-6]. In Egypt, tomatoes are known to be highly susceptible to root-knot nematodes Meloidogyne spp., caused annual losses of 12% [7].
The continuous use of chemical nematicides to control root-knot nematode had a considerable environmental impact and has resulted in the onset of resistance phenomena within some populations of nematode pests, this situation has led to an increased demand for environment-friendly products in order to reduce the effects of widespread nematicides utilization in crop protection [8].
Several studies reported the ability of certain plants to suppress nematodes through the nematicidal activity of the secondary metabolites [9,10]. Several researches on biofumigation had focused on using brassicaceous crops [11]. The suppressive effect of brassicaceous, biofumigants on Phyto-nematodes were explained in numerous studies under in vitro, in vivo, and field conditions [12-14]. The mechanism responsible for the biocidal effect of decomposing brassica crops was thought to be based on a chain of chemical reactions ultimately resulting in the formation of biologically active products [15]. Brassicaceae plants contain glucosinolate compounds, which are β-D thioglucosides, sulphur containing stable and non-toxic compounds located in the cell vacuoles distinguished from one another by differences in their organic side chains (R groups) and classified as aliphatic, aromatic or indole forms, occur in all parts of the plant and degrade via enzymatic hydrolysis [16]. The fumigant action of these volatile compounds that are released, suppresses plant pathogens soil-borne pathogens [17,18].
The aim of this study was to determine the biofumigation effects of fodder radish, and rocket salade on the root-knot nematodes, Meloidogyne spp., and tomato yield under field conditions in addition to identification of bioactive compounds of the two plants under investigation using Gas liquid chromatography-mass spectrometry (GLC-MS) and correlate between the glucosinolate autolysis products and nematicidal activity.
Materials and Methods
Source of plant seeds
Two brassica plants, i.e., fodder radish (Raphanussativus var. Terranovah-4-169/0300), was obtained from Joordens Zaden Company, Netherlands. Rocket salad(Eruca sativa cv. Baladi) was obtained from Horticultural Research Institute, Agriculture Research Center, Egypt, Giza.
Field experiments
The experiments were conducted at naturally infested with root-knot nematodes Meloidogyne spp., in the experimental farm of Faculty of Environmental Agricultural Sciences, Arish University during two successive seasons of (2017, and 2018) using the same field design plots of applied treatments. The experiments were divided into 16 plots, each plot (2.5 × 3.5 m2), all treatments were added in the two seasons as follows:
1. Biofumigation crops of fodder radish (Raphanussativus var. Terranovah).
2. Biofumigation crops rocket salad (Eruca sativa cv. Baladi ).
3. Nematicide treatment Vydate (oxamyl) 24% L.
4. Control treatment (without any treatment).
Preparation of soil
Sixteen individual plots were arranged 2.5 × 3.52 with the 1m interval between cross plots. Plots were plowed to 30 cm depth by the garden tiller. Seeds of (fodder radish, and rocket salad) were sterilized before using and sowed in arranging plots as a recommended amount (2.4kg/Fed) (15 December 2017). Vydate (oxamyl) 24% L as a nematicide was used at 4L/Fed (recommended dose) was used for comparison. Control treatment kept free without any treatment. The experiments were in a completely randomized block design with four replicates.
Incorporation of brassica plants and evaluation of their biofumigation effects on tomato plants
After 3 months (15 March 2018) at the full blooming stage (3 months after cultivation), 4 plants were randomly selected from each replicate. Shoots and roots were dried at 70˚C till a constant weight to determine both roots, shoot and whole plant dry weights (g/plant). After that, the dried plant materials were subject to the determination of C/N ratio of the whole plant.
Another 4 plants were used to determine nematicidal phytochemical profiles using GlC-MS. The brassica plants were incorporated into the top 25-30 cm of the soil by a garden tractor with a rotary tiller. The plots were irrigated to field capacity and covered with a transparent polyethylene film (50-micron thickness) in order to prevent any evolved gasses from escaping to the atmosphere, as well as, to increase the temperature to accelerate the decomposition process of brassica as biofumigation crops. Irrigation was continued at 3 day-interval during the decomposition period (four weeks). After that, plastic sheets were removed, and the soil was left for two weeks before tomato transplanting. Tomato seedlings were planted, and all soil partitions were planted at the same time with a tomato seedling (Solanum lycopersicum) cv. "Elisa"35 days old with 4-5 true leaves". Seedlings were transplanted in each plot at a distance of 40 cm in the row and 40 cm between rows. Tomato plants were grown by drip irrigation.
Three months after transplanting tomato seedlings, harvest was done and 10 tomato plants from each plot were randomly selected and the growth parameters were: shoot and root length (cm), fresh shoot and root weights (g) as well as dry weight (g). Also, fruit yield weighs (g) were recorded. Increasing percentages fresh weight of the whole plant, and fruit yield (g) % = Treatment - control/control × 100.
The roots of these plants were carefully removed and recorded numbers of galls, gall index, number of egg masses/ root system, as well as, number of juveniles in each plot [19]. Root galling was estimated according to [20], whereas: 0= no galls or egg-mass 1= 1-2 galls or egg-mass 2= 3-10 galls or egg-mass 3= 11-30 galls or egg mass 4= 31-100 galls or egg-mass 5= more than 100 galls or egg-mass. Egg-masses were stained prior to counting by dipping the infected roots in phloxine-B solution (0.015%) for 20 minutes as described by Daykin et al. [21]. Reduction percentages of root knot nematode galls, egg-masses numbers/ root system, and number of the secondstage juveniles (j2) in 250 g of soil were calculated in comparison with a control treatment.
Preparation of glucosinolates hydrolysis products by natural autolysis
Each plant under investigation (20 g fresh weight) was homogenized with distilled water (200 mL) and left for natural autolysis overnight (15 hours) at room temperature (≈25°C). Each mixture was shaken with dichloromethane (50 mL) for 20 min and centrifuged for 5 min at 3500 rpm. Anhydrous sodium sulfate was used to dry the separated organic layer which then concentrated under nitrogen to about 0.5 mL and kept (in a tightly closed vial) in deep freezer until analysis [22].
Gas Liquid Chromatography-Mass Spectrometry (GLCMS)
1μL of dichloromethane extract of each plant autolysate was injected into an Agilent 6890 gas chromatography (USA) equipped with PAS- 5MS capillary column (30 m × 0.32 mm; 0.25 μm film thickness), attached to an Agilent 5973 quadrupole mass spectrometer. The injector temperature was 250°C and the temperature program started at 45°C isothermal for 3 min and raised to final temperature 280°C at 5°C/min, 10 min isothermal. Helium was the carrier gas (1 ml/min). The mass spectrophotometry detector was operated in an electron impact ionization mode and ionizing energy of 70 eV scanning from m/z 40 to 500. The temperature of ion source was 230°C. The percentage composition was computed from the total ion chromatogram peak areas (Table 1).
| Brassica species | Root (g) | Shoot (g) | Whole plant (g) | Root/Shoot ratio |
Organic matter,% |
Total Nitrogen, % |
Organic Carbon,% |
C/N ratio | Protein % |
|---|---|---|---|---|---|---|---|---|---|
| R. sativus var. Terranovah | 9.32 | 39.22 | 48.54 | 0.24 | 29.1 | 3.10 | 33.3 | 10.1 | 19.4 |
| E. sativa cv. Baladi | 2.74 | 18.96 | 21.70 | 0.14 | 20.8 | 3.20 | 29.5 | 9.20 | 20.0 |
Table 1: Chemical analysis of two studied brassica species and its dry weight (g/plant).
Statistical analysis
All experiments were performed twice in a completely randomized design with three replicates in each treatment. Data were subjected to analysis of variance (ANOVA) using MSTAT-C program version 2.10 [23,24]. Means were compared by Duncan’s multiple range test at P ≤ 0.05 probability [25].
Results and Discussion
Effect on nematode population
Results obtained in both seasons 2017 and 2018 were almost identical Table 2 indicated that significant (p≤0.05) reduced nematode parameters on tomato plants, under field conditions. Average percentages of nematode population reduction were calculated compared with control treatment. In general the effect of Raphanus sativus var. Terranovah, as biofumigation crop recorded the average of the percentage reduction in number of galls, egg-masses/root system, and a number of second-stage juvenile (j2) /250 g of soil (84, 90, and 84%) respectively in season 2017. While, were (90,87, and 88%) in season 2018 respectively. On the other hand, Eruca sativa cv. Baladi as biofumigation crop recorded (75, and 82%) of the percentage reduction in number of galls, (55% and 73%) egg-masses/root system, and a number of second-stage juvenile (j2) /250 g of soil (77% and 75%) in seasons 2017 & 2018 respectively. The ability of certain plants to suppress nematodes through the nematicidal activity of the secondary metabolites has been reported [26]. Research has furthermore proved that many Brassicaceous species show nematicidal efficacy of plant-parasitic nematodes [27,28]. The plant species that generally are considered for biofumigation are found mostly in family Brassicaceae and include Eruca sativa (rocket salad), and Raphanus sativus (radish), [29,30]. The present results are agreemet with those obtained by Aydınlı et al. [31] reported that used radish (Raphanus sativus) and rocket salad (Eruca sativa) as biofumigation crops decreased gall index and egg masses of root-knot nematode M. arenaria on tomato plants under the greenhouse conditions. The same trend was noticed with El-Nagdi [19], who reported that used two mashed leaves of plants belonging to the genus brassica, cabbage (Brassica oleracea) and kohlrabi (Brassica caulorapa) were used as pre-and at sowing biofumigants for managing root-knot nematode, Meloidogyne incognita on cowpea plants, under greenhouse conditions. They found that the addition of the plant's residue cabbage and kohlrabi 10 days before sowing gave the highest percentages of final nematode reduction (70.4, and 82.1%) respectively. Also, when the C/N ratio of the amendment is less than 20:1, more effect on nematodes is evident [32,33]. On this basis, (R. sativus and E. sativa) residue used in this study, have a C/N ratio (10.1, and 9.20) so more effect of its toxic biofumigants on the nematode population.
| Season, 2017 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | No. galls/ root system | Reduction (%) | Root gall index | No. egg-masses/ root system | Reduction (%) | Egg- masses index | No. J2/250g of soil | Reduction (%) |
| R. sativus var. Terranovah | 13.3c | 84 | 3 | 4c | 83 | 2 | 78c | 84 |
| E. sativa cv. Baladi | 21.3b | 75 | 3 | 10.3b | 55 | 2 | 115b | 77 |
| Vydate (oxamyl) 24% L | 8c | 90 | 2 | 3c | 87 | 2 | 61.6c | 87 |
| Control (without any treatment) | 85.3a | - | 4 | 23a | - | 3 | 491.3a | - |
| Season, 2018 | ||||||||
| R. sativus var. Terranovah | 12c | 90 | 3 | 3.6c | 87 | 2 | 66 c | 88 |
| E. sativa cv. Baladi | 21b | 82 | 3 | 7.6b | 73 | 2 | 130.6b | 75 |
| Vydate (oxamyl) 24% L | 5.6c | 95 | 2 | 2c | 93 | 1 | 52c | 90 |
| Control (without any treatment) | 115.3a | - | 5 | 28a | - | 3 | 528a | - |
*Different letter (s) indicate significant differences among treatments within the same column according to Duncan's multiple range test (P ≤ 0.05). * Root gall index (RGI) or egg-masses index (EI) was determined according to Taylor and Sasser (1978) where G.I and E.I were determined as follows: 0: = no galls, 1= 1-2, 2 = 3-10, 3 = 11-30, 4 = 31-100, 5= more than 100 galls or egg- masses per root system.
Table 2: Effect of Brassica as biofumigation crops (R. sativus, and E. sativa) on root galling and nematode reproduction of Meloidogyne spp., under field conditions during successive seasons 2017 & 2018.
Effect on tomato growth and yield
As shown in Table 3 all plant growth parameters on tomato plants significant increased (p≤0.05) by using the tested treatments. The obtained results indicated that the effect of R. sativus as biofumigation crop recorded the average highest percentages. i.e., the plant growth vigor, increase, and fruit yield per plant compared with control treatment. The increase in fresh weight of whole tomato plant, and fruit yield were (57, and 92%), and (64, and 102%) in seasons 2017 &2018 respectively, this may be attributed to the relatively high biomass accumulation by fodder radish (48.54g/plant) Table 1. The same trend occurred for E. sativa as biofumigation crop increased in fresh weight of whole tomato plant, and fruit yield were (57, and 79%), and (46, and 93%) in seasons 2017 & 2018 respectively. The present results are agreement with those obtained by Anita [6] who, reported that ethanol extracts of cabbage, cauliflower, radish and chinese cabbage leaves reduced population of M. hapla and improved celery plant growth criteria, of which, radish leaf residue increase 41.9% in celery green leaves and stalk yield.
| Treatments | Shoot length (cm) | Root length (cm) | Shoot fresh weight (g) | Root fresh weight (g) | Fresh weight of whole plant (g) | Inc % | Shoot dry weight (gs) | Root dry weight (g) | Fresh fruit weight/ plant (g) | Inc% |
|---|---|---|---|---|---|---|---|---|---|---|
| Season, 2017 | ||||||||||
| R. sativus var. Terranovah | 56.7a | 33.6a | 287.8b | 72.6a | 360.4b | 57 | 91b | 33b | 448.6a | 92 |
| E. sativa cv. Baladi | 49.6b | 28 b | 264.6c | 65.6b | 330.2c | 57 | 77.6c | 27.3c | 418.3b | 79 |
| Vydate (oxamyl) 24% L | 58.2 a | 33a | 302a | 75a | 377a | 64 | 96.3a | 36.6a | 463a | 98 |
| Control (without any treatment) | 28.9c | 19.2c | 186d | 44c | 230d | - | 60.3d | 20d | 233.3c | - |
| Season, 2018 | ||||||||||
| R. sativus var. Terranovah | 64.3b | 37a | 285.6b | 77b | 362.6b | 64 | 94b | 34a | 491b | 102 |
| E. sativa cv. Baladi | 51c | 29.6b | 258.3c | 64c | 322.3c | 46 | 80.6c | 26.3b | 468.3c | 93 |
| Vydate (oxamyl) 24% L | 67.6a | 37a | 299.3a | 83a | 382.3a | 73 | 104a |
35a | 504a | 107 |
| Control (without any treatment) | 42.6d | 20.3c | 182.3d | 39d | 221.3d | - | 34.6d | 19.3c | 243d | - |
*Different letter (s) indicate significant differences among treatments within the same column according to Duncan’s multiple range test (P ≤ 0.05 Inc (%) = treatment - control / control × 100.
Table 3: Effect of Brassica as biofumigation crops (R. sativus and E. sativa) on the growth of tomato infected with Meloidogyne spp., under field conditions during successive seasons 2017 & 2018.
Gas liquid chromatography-mass spectrometry (GLC-MS) of R. sativus and E. sativa
Analysis of dichloromethane extract of fodder radish and rocket salad indicated the presence of glucosinolates hydrolysis products including isothiocyanate, nitriles and epithioalkane nitriles. Total ion current chromatogram of the GLC analysis of dichloromethane extract of autolysis products of fodder radish, and rocket salade, were shown in Tables 4 and 5, and Figures 1 and 2. The identification was based upon a comparison of mass fragments and their relative intensities with the available literature [23,34-37] and Wiley 9th edition NIST11 (W9N11) USA, database libraries.
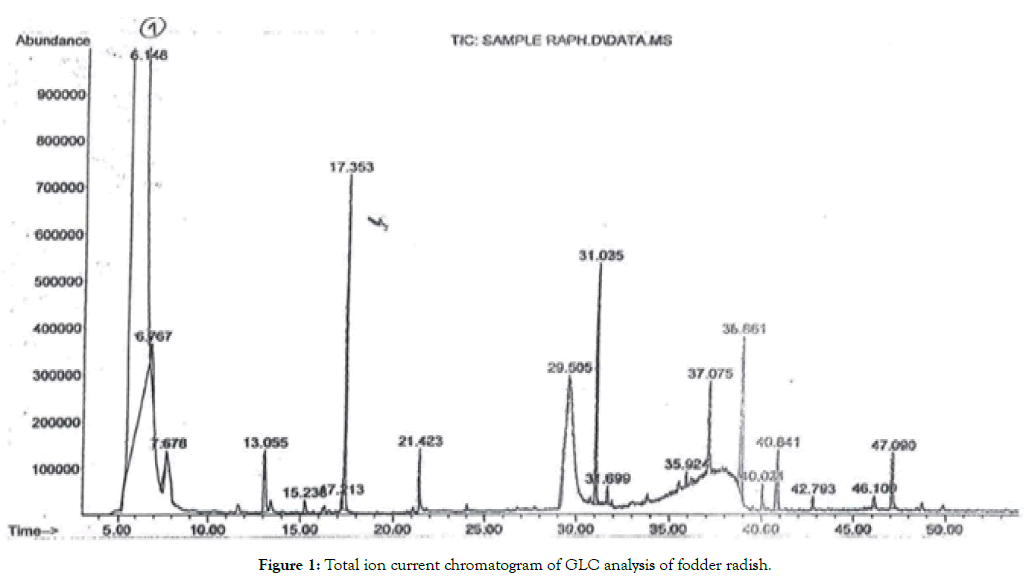
Figure 1: Total ion current chromatogram of GLC analysis of fodder radish.
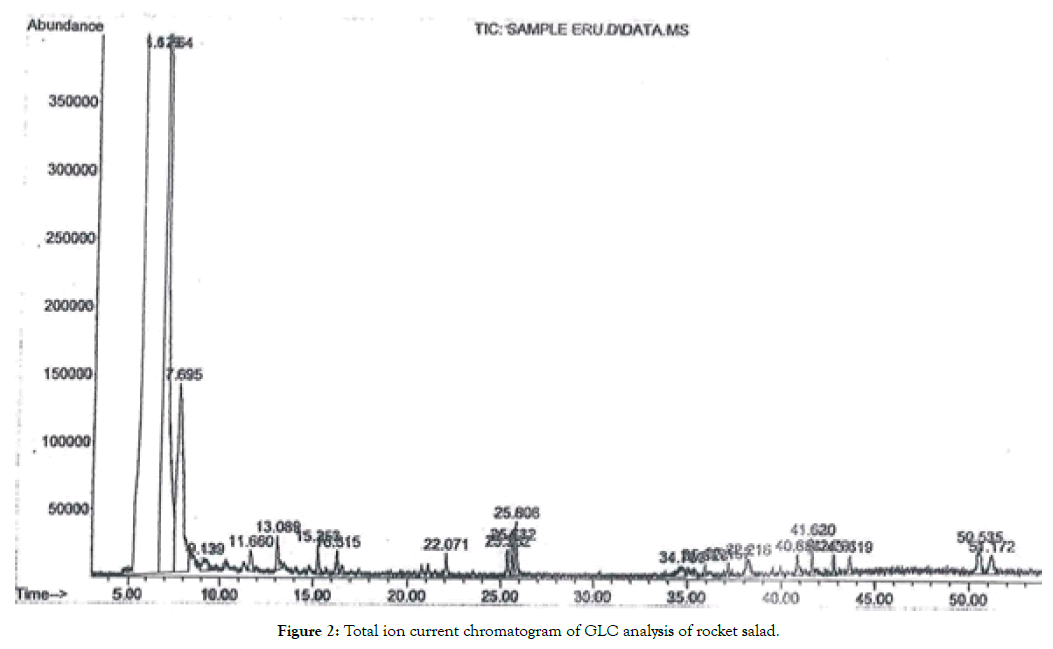
Figure 2: Total ion current chromatogram of GLC analysis of rocket salad.
| Parent compound Hydrolysis product | Rt (min) | Relative (%) of autolysis products | Major mass fragments* |
|---|---|---|---|
| Gluconapin 3- Butenyl isothiocynate |
17.35 | 14.15 | 113 (M+), 72 and 55 |
| Glucoerucin 4- (Methylthio) butyl Isothiocynate (erusin) |
31.033 | 9.4 | 161 (M+), 146, 115, 100, 72 and 61 |
| Glucoraphanin 4- (Methylsulfinyl) butyl isothiocynate (sulforaphane) |
38.85 | 10.37 | 177 (M+), 160, 114, 72 and 55 |
| Glucoerysolin 4- (Methylsulphony) butyl isothiocyanate (erysolin) |
40.02 | 9.4 | 193, 135, 114, 86, 72, 55 and 41 |
Table 4: Glucosinolates hydrolysis products obtained by natural autolysis of R. sativus var. Terranovah (Fodder radish).
| Parent compound Hydrolysis product |
Rt (min) | Relative (%) of autolysis products | Major mass fragments* |
|---|---|---|---|
| Gluconapin 4,5-Epithiopentanenitrile |
22.06 | 1.2 | 113 (M+), 86, 80, and 73 |
| Progoitrin (R) 1-cyano-2-hydroxy-3,4-epithiobutane (Threo) |
25.35 | 1.2 | 129 (M+), 96, 89, 83, 61, 55 and 40 |
| Gluconasturtiin 1-Benzenepropane nitrile |
25.63 | 1.2 | 131 (M+),91, 77, 65 and 52 |
| Epiprogoitrin (S) 1-cyano-2-hydroxy-3,4-epithiobutane (Erythro) |
25.81 | 1.2 | 129 (M+), 111, 96, 89, 83, 61, 59, 55 and 41 |
| Glucosativin 1,3-Thiazepane-2-thione, (4-Mercaptobutyl isothiocyanate) (sativin) |
38.21 | 2.38 | 175 (M+), 114, 87, 72 and 55 |
*: Base ion in bold
Bioactive compound names, retention time, relative percentage, and mass spectral data of each compound in each plant extract of glucosinolates hydrolysis products were listed in Tables 4 and 5.
Results in Figure 1, and Table 4, indicated that four glucosinolates were identified in fodder radish through their volatile autolysis products. Gluconapin, the major compound was identified by 3- butenyl isothiocynate while glucoerucin was identified by 4-(methylthio) butyl isothiocynate, which is commonly known as erucin. Sulphoraphane was released from 4-(methylsulfinyl) butyl glucosinolate (glucoraphanin) while 4-(methylsulphony) butyl isothiocyanate commonly known as erysolin was liberated from glucoerysolin.
The results indicated that rocket salad autolysate had five GLS Figure 2, and Table 5. Gluconapin was detected in rocket salad also and its presence was identified through its epithionitrile; 4,5-epithiopentanenitrile. The isomers progoitrin and epiprogoitrin were detected by the two hydrolysis products diastereomers threo and erythro 1-cyano-2-hydroxy-3,4-epithiobutane respectively. The aromatic glucosinolate; gluconasturtiin could be identified through its liberated nitrile named as 1-benzenepropane nitrile. Sativin, the major identified compound is 4-mercaptobutyl isothiocyanate liberated from glucosativin according to several researchers [38-40]. Fechner et al., [41] revealed that sativin was proved to be 1,3-thiazepane-2-thione, a tautomer of 4-mercaptobutyl isothiocyanate with cyclic 7-membered ring structure according to NMR data.
Gluconapin, glucosinolate (glucoraphanin) 4-(methylsulphony) butyl isothiocyanate. An alternative management strategy that is increasingly receiving interest is biofumigation that are a sustainable approach to manage soil-borne pathogens, nematodes, and weeds. This process was defined as a process that occurred, when volatile compounds with pesticidal properties were released during the decomposition of plant materials or animal products [42]. Some isothiocyanates such as benzyl isothiocyanate, methyl isothiocyanate, and phenyl isothiocyanate have been extracted from brassica species and showed nematicidal effects [43]. Other isothiocyanates also showed some nematicide effects, such as ethyl isothiocyanate benzyl thiocyanate, 1-phenylethyl isothiocyanate, and 2-phenylethyl isothiocyanate. However, acryloyl isothiocyanate and allyl isothiocyanate showed the highest effect against nematodes [44]. Glucosinolates and their derivatives, such as isothiocyanates, isolated from various brassica species differ in their toxicity against nematodes. Species containing benzyl or 2-phenylethyl and smaller level allyl isothiocyanate were more effective against Tylenchulus semipenetrans, and M. javanica ,when compared to butyl, ethyl, methyl, phenyl, and 4-methylsulfinyl isothiocyanate [45]. Glucosinolates are one of the most frequently studied groups of defensive secondary metabolites in plants. Upon cellular disruption, e.g. wounding by nematodes, the thioglucoside linkage is hydrolyzed by endogenous enzymes (myrosinases), resulting in the formation of products (e.g. isothiocyanate, thiocyanate, nitrile, epithionitrile, oxazolidine-2-thione) that are active against herbivores and pathogens [46,47]. For instance, glucosinates purified from Brassicaceae (Brassica napus, B. rapa, B. carinata, Lepidium sativum, Raphanus sativus, and Sinapis alba) were not toxic to j2s of the cyst nematode Heterodera schachtii in their original form, but enzymatic hydrolysis products of glucosinolates (isothiocyanate, sinigrin, gluconapin, glucotropeolin, dehydroerucin) were lethal to the nematode [48]. Similarly, 11 glucosinolates and their degradation products did not affect j2s of the root knot nematode M. incognita, but myrosinase hydrolysis products (gluconasturtiin, glucotropaeolin, glucoerucin, and sinigrin) were highly toxic [49]. Other studies also report that glucosinolates are only lethal to the cyst nematode Globodera rostochiensis in the presence of myrosinase [50].
Conclusion
From the obtained results, it can be concluded that (fodder radish, and rocked salad) when were utilized as biofumigation crops and compared to nematicide Vydate (oxamyl) 24% L, were effective in reducing population density of root-knot nematode Meloidogyne spp., infecting tomato plants and improved plant growth parameters. The highest percentage of the nematode population reduction occurred when of R. sativus as a biofumigation crop in two successive seasons 2017 & 2018 respectively.
REFERENCES
- FAO. FAOSTAT. Food and Agriculture Organization of the United Nations. Rome. 2019.
- FAO . FAOSTAT. Food and Agriculture Organization of the United Nations. Rome. 2016.
- Nicol JM, Turner SJ, Coyne DL, den Nijs LJ, Hockland S, Maafi ZT. Current nematode threats to world agriculture. In Genomics and molecular genetics of plant-nematode interactions. Springer, Dordrecht. 2011.
- Castagnone-Sereno P, Danchin EG, Perfus-Barbeoch L, Abad P. Diversity and evolution of root-knot nematodes, genus Meloidogyne: new insights from the genomic era. Ann Rev Phytopathol. 2013;51:203-220.
- Anes KM, Gupta GK. Distribution of plant parasitic nematodes in the soybean (Glycine max) growing areas of India. Indian J Nematol. 2014;44:227-231.
- Anita B. Crucifer vegetable leaf wastes as biofumigants for the management of root knot nematode (Meloidogyne hapla Chitwood) in celery (Apium graveolens L.). J Biopest. 2012;5:111.
- Abd-Elgawad M. Yield losses by phytonematodes: challenges and opportunities with special reference to Egypt. Egyptian J Agronematol. 2014 ;13(1):75-94.
- Salem MF, Mahdy ME. Suppression of root-knot nematode through innovative mustard biofumigation.
- Chitwood DJ. Phytochemical based strategies for nematode control. Ann Rev phytopathol. 2002;40:221-249.
- Zasada IA, Ferris H. Nematode suppression with brassicaceous amendments: application based upon glucosinolate profiles. Soil Biol Biochem. 2004;36(7):1017-1024.
- Kirkegaard JA, Matthiessen J. Developing and refining the biofumigation concept. Agroindustria. 2004;3(3):233-239.
- Ploeg A. Biofumigation to manage plant-parasitic nematodes. In: Integrated management and biocontrol of vegetable and grain crops nematodes. Springer, Dordrecht. 2008;239-248.
- Ploeg A, Stapleton J. Glasshouse studies on the effects of time, temperature and amendment of soil with broccoli plant residues on the infestation of melon plants by Meloidogyne incognita and M. javanica. Nematol. 2001;3(8):855-861.
- Zasada IA, Halbrendt JM, Kokalis-Burelle N, LaMondia J, McKenry MV, Noling JW. Managing nematodes without methyl bromide. Ann Rev phytopathol. 2010;48:311-328.
- Underhill EW. Glucosinolates. In: Bell EA and Charlwood BV (eds.) Encyclopaedia of Plant Physiology Vol 8 Secondary Plant Products. Springer-Verlag, Berlin, Germany. 1980;7493-511
- Padilla G, Cartea ME, Velasco P, de Haro A, Ordás A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochem. 2007;68(4):536-545.
- Kirkegaard JA, Sarwar M. Biofumigation potential of Brassicas. Plant and soil. 1998;201(1):71-89.
- Buena AP, García-Álvarez A, Díez-Rojo MA, Ros C, Fernández P, Lacasa A, et al. Use of pepper crop residues for the control of root-knot nematodes. Bioresource Technol. 2007;98:2846-2851.
- Goodey T. Laboratory methods for work with plant and soil nematodes. Technical Bulletin. Ministry of Agriculture and Fisheries. 1949.
- Taylor AL, Sasser JN. Biology, identification and control of root-knot nematodes. North Carolina State University Graphics. 1978;111.
- Daykin, M. E., and Hussy, R. S. (1985).Staining and histopathological techniques in nematology.Pages 19-35 in: An Advanced Treatise on Meloidogyne. Vol. 1. Biology and Control. Barker K. R.,. Carter C. C, and Sasser JN (eds.) North Carolina State University, Raleigh.
- Al‐Gendy AA, Lockwood GB. GC–MS analysis of volatile hydrolysis products from glucosinolates in Farsetia aegyptia var. ovalis. Flavour Frag J. 2003;18(2):148-152.
- Anonymous SA. A Software Program for the MSTAT-C Design, Management and Analysis of Agronomic Research Experiments. Michigan State Univ. 1991.
- Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11(1):1-42.
- Ntalli NG, Caboni P. Botanical nematicides: A review. J Agri Food Chem. 2012;60(40):9929-9940.
- Monfort WS, Csinos AS, Desaeger J, Seebold K, Webster TM, Diaz-Perez JC. Evaluating Brassica species as an alternative control measure for root-knot nematode (M. incognita) in Georgia vegetable plasticulture. Crop Protect. 2007;26(9):1359-1368.
- Van Dam NM, Tytgat TO, Kirkegaard JA. Root and shoot glucosinolates: a comparison of their diversity, function and interactions in natural and managed ecosystems. Phytochem Rev. 2009;8(1):171-186.
- Ploeg AT. Biofumigation to manage plant-parasitic nematodes. In: Ciancio A & Mukerji, KG (eds) Integrated management and biocontrol of vegetable and grain crops nematodes. Springer-Verlag, Berlin. 2007;239–248.
- Lopez-Perez JA, Roubtsova T, de Cara García M, Ploeg A. The potential of five winter-grown crops to reduce root-knot nematode damage and increase yield of tomato. J Nematol. 2010;42(2):120.
- Edwards S, Ploeg A. Evaluation of 31 potential biofumigant brassicaceous plants as hosts for three Meloiodogyne species. J Nematol. 2014;46(3):287.
- Aydınlı G, Mennan S. Biofumigation studies by using Raphanus sativus and Eruca sativa as a winter cycle crops to control root-knot nematodes. Brazilian Biol Technol. 2018;61.
- Youssef MM. Brassica vegetable leaf residues as promising biofumigants for the control of root knot nematode, Meloidogyne incognita infecting cowpea. Agri Eng Int CIGR J. 2019;21(1):134-139.
- Stirling GR. Biological control of plant-parasitic nematodes. CRC Press Inc. 1988.
- Spencer GF, Daxenbichler ME. Gas chromatography‐mass spectrometry of nitriles, isothiocyanates and oxazolidinethiones derived from Cruciferous glucosinolates. J Sci Food Agri. 1980;31(4):359-367.
- Blažević I, Mastelić J. Glucosinolate degradation products and other bound and free volatiles in the leaves and roots of radish (Raphanus sativus L.). Food Chem. 2009;113(1):96-102.
- Blažević I, Radonić A, Mastelić J, Zekić M, Skočibušić M, Maravić A. Glucosinolates, glycosidically bound volatiles and antimicrobial activity of Aurinia sinuata (Brassicaceae). Food Chem. 2010;121(4):1020-1028.
- Mohammed ED, El-Naga RN, Lotfy RA, Al-Gendy AA, El-Demerdash E. Anti-fibrotic potential of a Matthiola arabica isothiocyanates rich fraction: Impact on oxidative stress, inflammatory and fibrosis markers. Die Pharmazie-An Int J Pharm Sci. 2017;72(10):614-624.
- Bennett RN, Mellon FA, Botting NP, Eagles J, Rosa EA, Williamson G. Identification of the major glucosinolate (4-mercaptobutyl glucosinolate) in leaves of Eruca sativa L.(salad rocket). Phytochem. 2002;61(1):25-30.
- Cataldi, Tommaso RI, Alessandra Rubino, Filomena Lelario, and Sabino A. Bufo. "Naturally occurring glucosinolates in plant extracts of rocket salad (Eruca sativa L.) identified by liquid chromatography coupled with negative ion electrospray ionization and quadrupole ion‐trap mass spectrometry." Rapid Communications in Mass Spectrometry: An International Journal Devoted to the Rapid Dissemination of Up‐to‐the‐Minute Research in Mass Spectrometry 21. 2007: 2374-2388.
- Jasper J, Wagstaff C, Bell L. Growth temperature influences postharvest glucosinolate concentrations and hydrolysis product formation in first and second cuts of rocket salad. Post Biol Technol. 2020;163:111157.
- Fechner J, Kaufmann M, Herz C, Eisenschmidt D, Lamy E, Kroh LW, et al. The major glucosinolate hydrolysis product in rocket (Eruca sativa L.), sativin, is 1, 3-thiazepane-2-thione: Elucidation of structure, bioactivity, and stability compared to other rocket isothiocyanates. Food Chem. 2018;261;57-65.
- Kirkegaard JA, Sarwar M, Matthiessen JN. Assessing the biofumigation potential of crucifers. In International Symposium Brassica 97, Xth Crucifer Genetics Workshop 459. 1998;105-112.
- Aissani N, Tedeschi P, Maietti A, Brandolini V, Garau VL, Caboni P. Nematicidal activity of allylisothiocyanate from horseradish (Armoracia rusticana) roots against Meloidogyne incognita. J Agri Food Chem. 2013;61(20):4723-4727.
- Wu H, Wang CJ, Bian XW, Zeng SY, Lin KC, Wu B, et al. Nematicidal efficacy of isothiocyanates against root-knot nematode Meloidogyne javanica in cucumber. Crop protect. 2011;30:33-37.
- Zasada IA, Ferris H. Sensitivity of Meloidogyne javanica and Tylenchulus semipenetrans to isothiocyanates in laboratory assays. Phytopathol. 2003;93(6):747-750.
- Lambrix V, Reichelt M, Mitchell-Olds T, Kliebenstein DJ, Gershenzon J. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell. 2001;13(12):2793-807.
- Santolamazza-Carbone S, Velasco P, Soengas P, Cartea ME. Bottom-up and top-down herbivore regulation mediated by glucosinolates in Brassica oleracea var. acephala. Oecologia. 2014;174(3):893-907.
- Lazzeri L, Curto G, Leoni O, Dallavalle E. Effects of glucosinolates and their enzymatic hydrolysis products via myrosinase on the root-knot nematode Meloidogyne incognita (Kofoid et White) Chitw. J Agri Food Chem. 2004;52(22):6703-6707.
- Buskov S, Serra B, Rosa E, Sørensen H, Sørensen JC. Effects of intact glucosinolates and products produced from glucosinolates in myrosinase-catalyzed hydrolysis on the potato cyst nematode (Globodera rostochiensis cv. Woll). J Agri Food Chem. 2002;50(4):690-695.
- Aires A, Carvalho R, Barbosa MD, Rosa E. Suppressing potato cyst nematode, Globodera rostochiensis, with extracts of Brassicacea plants. Am J Pot Res. 2009;86(4):327-333.
Citation: Hassan S, Al-Gendy AA, Abdel-Baset SH, Abd El-Kareem SM, Massoud S, Abdallah M (2020) Biofumigation Potentials of Some Brassica Crops to Control Root-knot Nematodes Meloidogyne spp., on Tomato under Field Conditions. J Plant Pathol Microbiol. 11:527.
Copyright: © 2020 Hassan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

