Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2023) Volume 13, Issue 2
Azoximer Bromide as an Activator of Cellular Immunity: New Opportunities for Practical Use in Cancer Patients
L Yu Grivtsova1*, NA Falaleeva2 and NN Tupitsyn32Department of Drug Therapy, National Medical Radiology Research Centre, Moscow, Russia
3Department of Immunology, National Medical Research Center of Oncology, Moscow, Russia
Received: 26-Nov-2020, Manuscript No. JSCRT-20-7305; Editor assigned: 01-Dec-2020, Pre QC No. JSCRT-20-7305(PQ); Reviewed: 15-Dec-2020, QC No. JSCRT-20-7305; Revised: 31-Jan-2023, Manuscript No. JSCRT-20-7305(R); Published: 28-Feb-2023, DOI: 10.35248/2157-7633.23.13.579
Abstract
In this review, we have summarized the data on the new properties and uses of Azoximer Bromide (AB) in cancer patients. Recent studies have shown that AB, an immunomodulator with immunoadjuvant properties, can have a direct antitumor effect through a number of mechanisms leading to the activation of both cellular and humoral immune responses. Azoximer bromide mediates the transcription of cytosolic helicase receptor MDA5 gene and acts as a modifier of the innate immune response and inducer of the RIG-I/MDA5 signaling pathway, mainly due to its effect on MDA5. AB inhibits the accumulation of the Myeloid-Derived Suppressor Cells (MDSC) population in experimental aseptic inflammation conditions. AB reduces extracellular neutrophil traps formation in in vitro studies. Azoximer bromide enhances the expression of the costimulatory molecule ICOSL by 1.7 and increases the ability of dendritic cells to stimulate the maturation of follicular helper T-lymphocytes and enhance the T-dependent humoral response. Thus, AB should be considered as a drug with a direct antitumor effect, which is confirmed by a number of clinical studies and observations.
Keywords
Azoximer bromide; Immunotherapy; Cancer; MDA5; Extracellular neutrophil traps; MDSC
List of Abbreviations
AB: Azoximer Bromide; DAMP: Damage Associated Molecular Patterns; MDA5: Melanoma Differentiation Associated protein 5; MDSC: Myeloid Derived Suppressor Cells; PRR: Pattern Recognizing Receptors; TLR: Toll Like Receptors; РАМР: Pathogen Associated Molecular Patterns
Introduction
RIG-I/MDA5 and ICOS-ICOSL signaling pathways, NETosis inhibition in cancer patients and new role of azoximer bromide
Modern oncological practice uses a range of complex approaches aimed at the maximum possible tumor destruction including chemoradiation, surgical treatment and target therapy. The continuous process of research in the area of malignancies treatment has shifted the focus to immunotherapy based not on the tumor cell destruction but on modulating the work of all immune system components including tumor microenvironment. The purpose of these studies is to achieve complete cancer eradication. Recently, it has been demonstrated that the body can use the same signaling pathways for both antiviral and anticancer responses, i.e., in response to a tumor, the body activates the same defense systems as in response to viral infection, involving both specific and non specific mechanisms (Figures 1 and 2).
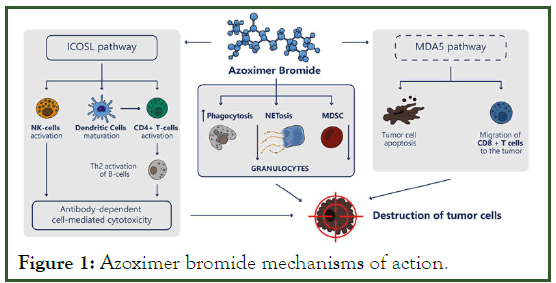
Figure 1: Azoximer bromide mechanisms of action.
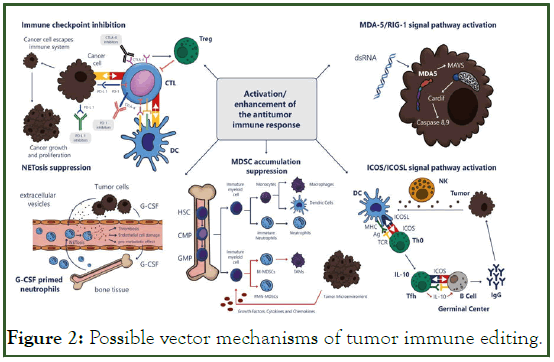
Figure 2: Possible vector mechanisms of tumor immune editing.
However, it is the innate immune system that plays a major role in the first line of defense against the pathogen by recognizing Pathogen Associated Molecular Patterns (РАМР) or Damage Associated Molecular Patterns (DAMP). PRR (Pattern Recognizing Receptors) act as the host structures that recognize, initiate, and regulate the innate immune response. They include TLR (Toll Like Receptors), membrane proteins (scavenger receptors and C-type lectins), secretory molecules (complement factors, acute phase proteins), cytoplasmic sensors (NOD, NALP, NAIP proteins), and cytoplasmic viral nucleic acid (RLR) receptors such as RIG-1, MDA5, and DAI. These structures initiate the activation of NF-kB, type I interferons, or other inflammatory signaling pathways. This leads to secretion of proinflammatory cytokines and chemokines and subsequent activation of cytotoxic immune cells, such as NK-cells and activated (CD8+) T-cells, which in turn trigger cytotoxic cell lysis and apoptosis [1].
Activation of RIG-I/MDA5 signaling pathways not only triggers anticancer immunity via IFN-dependent effector T-cells activation, but can also directly induce cancer cells apoptosis via IFN-independent route. Despite the common character of helicase receptors, there are reasons to believe that MD5 predominantly activates IFN-independent apoptosis. Selective effect of activating RIG-I/MDA5 signals on cancer and healthy tissue cells has been demonstrated; cancer cells are very sensitive to RIG-I/MDA5-induced apoptosis, while healthy tissue cells are protected by endogenous Bcl-xL activation. Therefore, triggering RIG-I/MDA5 activation using RIG-I/MDA5 ligands can induce tumor apoptosis. It is important that the activation of RIG-I/ MDA5 mediated apoptosis is not dependent on the p53 mutation presence in the tumor, thus no resistance will develop otherwise typical for chemoradiation exposure.
Tumor cells susceptibility to pro apoptotic signals of helicase containing cytoplasmic receptors (RLH-induced apoptosis) was demonstrated in the experiment on melanoma cells. Activation of the MDA5/MAVS/IRF7 signal pathway by 5-azacitidine (DNA demethylating target agent, 5-AZACdR) mediated interferon 1-dependent cell death in colorectal cancer cells. Stimulation of MDA5 and RIG-I receptors with respective ligands p (I: C) and 3pRNA caused apoptosis of a heterogeneous population of glioblastoma cells, including subpopulation of tumor initiating stem cells. At the same time, a synergy of MDA5 and TLR3 tumor suppression effects was established and MDA5 overexpression was associated with decreased survival of neuroblastoma cells in response to poly (I:C) in culture. Increased MDA5 gene expression or its ectopic expression can also mediate the death of cancer cells, in particular in early prostate cancer. One of the specific T-cell activation molecules is the ICOS molecule, which belongs to the immunoglobulin domain superfamily together with CD28/B7/CTLA-4 proteins.
Unlike the CD28 antigen, which is constitutively expressed on T-cells and provides the co-stimulating signals necessary for the complete activation of resting T-cells, ICOS is expressed only after activation. The molecule is involved in various aspects of Tcell and T-dependent humoral responses. Its critical role in the functioning of follicular T-helper cells, formation of germinal centers, regulation of Th2-cytokine production, including IL-10, interaction of T- and B-cells and the immunoglobulin class switching has been shown. ICOS+T cells may participate in autoimmune responses, including graft rejection reactions. In addition, the molecule is associated with the functioning of Tregulatory cells. Thus, as a result of the interaction between ICOS and its ligand expressed by cancer cells (in particular, by melanoma cells), naive T-cells are differentiated into IL-10 producing T-regulatory cells. These data confirm the important role of the ICOS molecule in the anticancer response [2].
It was shown that ICOS-L overexpression is generally associated with cancer progression and decreased overall survival rates. However, a dualistic effect of the ICOS/ICOS-L signal pathway is possible. For example, in colon cancer, an activation of this co-stimulating signal is associated with improved survival of patients. It is interesting to note that after the use of anticancer vaccine or after anti-CTLA-4 immunotherapy the number of peripheral ICOS+ T-cells significantly increased both in CD4+ and CD8+ populations. And the number of effector T-cells in tumor microenvironment also increased when compared to regulatory T-cells. There are evidences of mediating tumor growth in breast cancer via tumor infiltrating T-regulatory cells (T-regs) with ICOS expression and plasmacytoid dendritic cells expressing the ICOS ligand. In vitro experiments have shown that the addition of neutralizing anti-ICOS antibody has blocked T-regulatory cell expansion induced by plasmacytoid dendritic cells and interleukin-10 secretion by CD4(+) memory T-cells, which confirms the ICOS key role in this process. Also, the presence of ICOS(+) cells in breast cancer clinical specimens correlated with poor prognosis. This suggests the potential role of agents affecting ICOS/ICOS-L signal pathway as candidates for new effective anticancer therapy regimens. Non-clinical studies have shown that monoclonal Antibodies ICOS agonists (mAb) enhance immunotherapy effect seen with immune checkpoints inhibitors. Anti-ICOS antibodies can not only inhibit lymphoid tumor cells expressing ICOS, but can also weaken the immunosuppressive effect of T-regulatory cells. Currently, it is known that phase I/II clinical trials of two monoclonal antibodies-ICOS agonists and one antagonist are ongoing.
Literature Review
Netosis
One of the important links of non specific immune protection is granulocytes and their most numerous pool neutrophils, rapidly reacting to external threats. In recent years, much attention has been given to the study of neutrophils in cancer patients. To date, it has been shown that these blood cells are heterogeneous, contain at least 3 different fractions and moreover they have some functional and phenotypic plasticity like, for example, hematopoietic stem cells or macrophages. There are mature segmentonuclear High Density Neutrophils (HDN), Low Density Neutrophils (LDN) that accumulate in cancer patients, and immature granulocytic MDSC. HDN under the effect of a number of factors are capable of switching to LDN. This switching is accompanied by the loss of anticancer properties and the acquisition of immunosuppressive capacity typical for immature granulocytic MDSC. Transforming Growth Factor Beta (TGF-β) was identified as the main trigger. However, in experimental models, the effect of TGF-β alone did not have any effect on the ratio of high and low density neutrophil subpopulations, which indicates the existence of additional transition inducers [3]. Both LDN and MDSC have immunosuppressive properties and contribute to tumor progression and metastasis. HDNs function is toensure phagocytosis and cytotoxicity are carried out, while LDNs are responsible for NETosis, meaning the actively form Neutrophil Extracellular Traps (NETs). Currently, these structures, along with degranulation and phagocytosis, are considered as the third anti infective mechanism of neutrophils. In cancer patients NETs determine a poor prognosis, being one of the factors contributing to the delayed relapses and the development of metastatic disease. In the experiment it was found that NETs formed during inflammation contribute to the activation of resting tumor cells. Role of tumor induced bone marrow neutrophils and NETs formed by them in turn has been determined in premetastatic niches formation, particularly in ovarian cancer, including the early stages of the process. In addition, a reliable relationship between NETs formation and thrombogenesis in an experimental model of breast cancer has been shown. Detailed mechanisms of NETosis implementation are described in several publications. Thus, NET formation and NETosis are undesirable events in a cancer patient and it is necessary to search for strategies aimed at inhibiting these factors.
Myeloid derived suppressor cells
In addition to neutrophils other types of myeloid cells, namely monocytes/macrophages and dendritic cells, are important for the implementation of a full-fledged immune response.
Myelopoiesis involves differentiation of a multipotent progenitor cell and a myeloid oligopotent progenitor cell into unipotent monocytes, granulocytes and DC. Monocytes can later migrate to tissues, where they differentiate into macrophages and DCimportant immune effector cells. Immature Myeloid Cells (IMC), i.e., myeloid progenitor cells do not have an immunosuppressive function and are constantly present in small amounts in healthy people bone marrow. In chronic inflammatory processes, malignant neoplasms, chronic infections and autoimmune diseases, IMC differentiation is significantly reduced and accumulation of MDSC myeloid derived suppressor cells occurs.
The formation and accumulation of MDSCs in cancer is facilitated by various factors produced by tumor cells, such as prostaglandin E2 (PGE2), IL-6, IL-10, IL-1β, transforming growth factor (TGF)-β, as well as Stem Cell Factor (SCF) and proangiogenic factors, such as VEGF [4]. Cancer cells are able to release these factors not only in the form of soluble molecules, but also through exosomes. Taking up of exosomes containing PGE2 and TGF-β by bone marrow immature myeloid precursors leads to their transformation into immunosuppressive MDSCs.
MDSCs have a powerful immunosuppressive effect, promoting cancer progression through a number of mechanisms. Thus, MDSC are able to secrete TGF-β and IL-10, thereby mediating the generation of de novo FoxP3+ T-reg cells in vivo, regardless of the production of Nitric Oxide (NO). It was found that MDSC induce expression of members of the B7 family of immunoregulatory ligands, including B7-H1 (also known as the programmed cell death ligand 1 (PD-L1)), B7-H3 and B7-H4. Also, the recruitment of specific T-reg CCR5+ that promote tumor progression in melanoma was mediated by CCR5, CCL3, CCL4 and CCL5 ligands that are produced by intra-tumor MDSCs.
In order to stimulate T-regs, MDSCs induce the formation of macrophages with a M2-like phenotype that have immunosuppressive effect and do not produce IL-12, promoting tumor growth.
Spleen MDSC cause suppression of L-selectin, cell adhesion molecule on CD4+ and CD8+ T-cells and B-cells in the spleen, which leads to decreased targeted homing and antigendependent activation of cytotoxic CD8+ T-cells in lymph nodes.
MDSCs secrete Reactive Oxygen Species (ROS), which are toxic to most cell types and therefore contribute to tumor progression by destruction of tumor infiltrating cytotoxic lymphocytes.
In addition, MDSCs are able to deplete cysteine, an amino acid involved in the generation of glutathione, an antioxidant molecule that protects cells from free radicals, including ROS. MDSCs can also reduce resistance of immune cells to ROS.
Another mechanism used by MDSC to inhibit T-cell functions involves the formation of adenosine from ATP driven by CD39. Ectonucleotide triphosphate diphosphohydrolase 1 (ENTPDase1, CD39) converts ATP released into the extracellular space to AMP, before ecto-5’-nucleotidase (Ecto5'NTase, CD73) catalyzes its dephosphorylation into adenosine. Extracellular adenosine inhibits priming of naive T-cells, preventing Zap70, ERK and Akt phosphorylation and reducing the expression of effector molecules such as CD95L, perforin, IFN-γ, TNF-α and CD25, on activated T-cells. Tumor TGF-β induces CD39 and CD73 expression on MDSC isolated from peripheral blood and tumors of patients with non-small cell lung cancer in a HIF-1α- dependent manner, which leads to the accumulation of immunosuppressive adenosine and contributes to further tumor progression.
It was also found that tumor associated PMN-MDSCs express higher levels of PD-L1 than their circulating analogues. However, PD-L1 expression on PMN-MDSC was found to be higher in unresponsive melanoma patients treated with ipilimumab compared to responders [5]. Low levels of MDSC before ipilimumab treatment (CTLA-4 inhibitor) were associated with higher survival in patients with advanced melanoma, indicating a predictive role of MDSCs for immune checkpoint inhibitor therapy. Ex vivo PD-L1 induction on IMC is mediated by soluble M-CSF and VEGF factors. In a murine model of colorectal cancer, PD-L1 induction in MDSCs can be significantly reduced after IFN-γ neutralization.
The number of clinical and non-clinical studies evaluating the safety and efficacy of MDSC inhibition alone or in combination with radiation therapy, chemotherapy, surgery, or various types of immunotherapy used against cancer is steadily increasing. Possible therapeutic options include depletion of MDSCs, reduction of their immunosuppressive potential, prevention of their mobilization into the tumor, and modulation of myelopoiesis at the level of normal myeloid progenitor cells. Despite promising non-clinical data, further clinical studies are required to determine the optimal strategy of neoplastic process management at the MDSC level.
Analyzing the mechanisms described above, it could be stated that a new optimum strategy for cancer immunotherapy is being formed, aimed on modification of the main signaling pathways of the innate and cell-mediated immune responses. This can be achieved by enhanced expression of cytoplasmic proteins, particularly MDA5, as well as by inhibition/activation of the ICOS-ICOSL signaling pathway, neutrophil flexibility for possible blocking the NET formation and targeted impact on the immunosuppressive cell populations such as MDSC.
New properties of azoximer bromide
In the light of the above, the ideal drug product is the one that can stimulate the RIG-I/MDA5 signaling pathways, and in a greater degree MDA5, and simultaneously reduce or block the negative effects of neutrophils while maintaining their positive properties, as well as have an activating effect on the ICOS/ ICOSL signaling pathways, on the interaction of dendritic cell with naive T-cells and reduce the content of myeloid derived suppressor cells in the blood and the tumor microenvironment.
In this regard data on azoximer bromide marketed under the trade name Polyoxidonium (PO) looks very interesting. This drug product was initially developed and successfully used as an effective agent for infectious and inflammatory diseases of viral, bacterial and fungal origin. Immunoadjuvant properties of AB made it possible to develop an effective combined influenza vaccine Grippol+ and Grippol Quadrivalent, which does not contain viral RNA and includes influenza A and B viral antigens, as well as azoximer bromide as an immunoadjuvant [6].
AB is a physiologically active compound which belongs to a novel class of heterochain aliphatic polyamines that poses significant clinical interest. By its chemical structure, AB is a copolymer of N-oxidized 1,4-ethylene piperazine and (Ncarboxyethyl)- 1,4-ethylene piperazine bromide. AB is a freely water soluble biodegradable compound with molecular weight of 60 kDa-100 kDa. The drug product is approved in Russia as a vaccine adjuvant that stimulates the production of antibodies and is also registered for use in cancer patients. Copolymer chains are cleaved and easily eliminated from the body, which determines a low level of toxicity and a good safety profile. High safety level is confirmed by extensive post-marketing study in the territory of the European Union (Slovakia). High safety of AB was also demonstrated in combination with antigens in a commercial vaccine against influenza in analysis including about 50 million recipients on the territory of the Russian federation.
AB binds to human peripheral blood monocytes and neutrophils and to a lesser extent to lymphocytes, more over the drug product also has immunogenic properties. These include stimulation of IL-6 production, increasing bactericidal activity of white blood cells, activating the production of H2O2, and improving the ability of neutrophils and macrophages to engulf and process various infectious agents, including bacteria such as staphylococci, by about 40%-60%. This may explain the ability of AB to improve the resistance of human body to infectious agents. Until recently, data on the effect of AB on lymphocytes and their subpopulations were limited, but it was assumed that its immunomodulatory functions could partially improve antigen presentation, contributing to more active production of antibodies.
Alexia C, et al. investigated the effects of AB in vitro to identify its cellular targets on three different types of immune cells that play an important role in immune surveillance in cancer, namely Dendritic Cells (DC), T-cells and NK cells. The authors of the study found that AB effect was moderate, but highly consistent. Thus, a distinct effect of the drug product on some markers of dendritic cell activation was revealed and the possibility of maturation of a population of immature dendritic cells into their mature fractions under the effect of the drug product was shown. Moreover, in vitro AB use contributed to a significant expansion of CD4+ T-cells. These data suggest a possible role of the drug product in mediating both humoral and cell-mediated immunity by activating the cytotoxic response of lymphocytes via DC. But the effect on the NK cell fraction was minor. In addition, the immunomodulatory properties of AB varied between donors. Based on this study, the authors concluded that further study of the immune mechanisms of AB is necessary for its use as a promising drug product in the development of new immunotherapy clinical protocols.
Recently, a study was conducted to analyze the effect of influenza vaccines on the expression of innate immunity receptor (TLR/RLR) genes. In this study, the expression of genes from a group of toll-like receptors (TLR 2, 3, 4, 7, 8, 9) and genes of two cytosolic receptors of viral nucleic acids RIG-I and MDA5 on the acute monocytic leukemia cell line (THP-1) was studied. The purpose of this study was to evaluate the possibility of using the THP-1 cell line as a model for investigation of inactivated influenza vaccine activity at gene level. During the study, tumor cells were incubated with 0.2 ml of Grippol (Russia) and Vaxigrip (France) vaccines, and expression of innate immune receptor genes was evaluated using RT-PCR method. It was found that the split-vaccine Vaxigrip containing components of destroyed virions of influenza A and B viruses induced genes of type 3, 7 and 8 toll-like receptors (TLR 3, 7, 8) and the gene of the cytosolic helicase receptor RIG1. While the influenza trivalent polymer-subunit vaccine Grippol, containing surface antigens of influenza viruses (hemagglutinin and neuraminidase) types A and B together with azoximer bromide as immunoadjuvant, in addition to activating the expression of TLR 3, 7, 8 and RIG1 genes, also activated TLR 4 and resulted in multiple stimulation of the MDA5 gene transcription.
Since the main difference between Vaxigrip and Grippol formulations is the presence of AB in the latter, it has been suggested that it azoximer bromide mediates the transcription of the cytosolic helicase receptor MDA5 gene. These data allow to consider azoximer bromide as a possible modifier of the innate immune response and as an inducer of the RIG-I/MDA5 signaling pathway, mainly due to its effect on MDA5.
Another very important, recently identified property of AB is its ability to reduce the level of NET formation in experimental studies in vitro. In this study, human neutrophils were stimulated with 30 nM Phorbol-12-Myristate-Acetate (PMA), a known activator of NETosis. During the preliminary one-hour incubation of neutrophils with azoximer bromide and subsequent stimulation of PMA, the number of NETotic cells was significantly reduced. The range of effective AB doses was 100 μg/ml-1000 μg/ml. It was identified that the drug product does not reduce the number of late NETotic cells, but inhibits NETosis in its intermediate stages, when the cell membrane becomes loose and swells, but then the process stops, DNA release does not occur, and the neutrophil trap is not formed [7].
In addition to the described properties of azoximer bromide in the report study of Grippol Quadrivalent vaccine effect on functional properties of dendritic cells it was shown that azoximer bromide enhances the expression of costimulatory molecule ICOSL which plays an important role in the humoral immune response by 1.7 times potentially enhancing the ability of dendritic cells to stimulate maturation of follicular T-helper cells. Moreover, it was shown that azoximer bromide leads to the secretion of cytokines by T-helper cells of the second type, including interleukin 5, which confirms the data of French colleagues on the possibility of humoral immunity stimulation by PO resulting in antibody-dependent cell-mediated cytotoxicity.
No less significant was an experimental study that demonstrated that AB restricts the accumulation of MDSC populations in conditions of aseptic turpentine-induced inflammation, associated with the use of vanadium and chromium salts. The results of investigation of spleen cell populations from laboratory animals with His48+/CD11b/c+(MDSC) phenotype, have shown that after AB correction therapy spleen cellularity in rats did not change during the 1st day of aseptic inflammation development. The seventh day of inflammation development in all groups of animals was accompanied by a well-marked accumulation of MDSC in the spleen (Ñ?Ë?0.05). By the 14th day, MDSC content under AB influence remained at the level of 7th day, statistically significantly different from the control group animals that did not receive the drug (by 28%, Ñ?Ë?0.05). IFNγ- dependent Th1 immune response was established for both untreated animals and rats treated with AB. Under the influence of AB, the inflammatory process was positively resolved (leveled off) within 2 weeks.
Based on the analysis of literature data, we can conclude that the drug product azoximer bromide (trade name polyoxidonium) has a complex effect on the immune system. This effect results in a significant increase of the expression of the induced costimulatory molecule ICOS ligand by 1.7 times, thus increasing the ability of dendritic cells to stimulate maturation of T-follicular CD4+ cells and enhance humoral response and may contribute to the mechanism of antibodydependent cell-mediated cytotoxicity. Its effect on the cytosolic protein MD5 leads to stimulation of tumor cell apoptosis via innate immune cells. In addition, the drug product increases phagocytosis and reduces the ability of neutrophils to form extracellular neutrophil traps. Thus, PO is a drug product affecting the main mechanisms of innate immunity involved in the anticancer protection and can be considered as a promising drug product for improving the efficacy of therapeutic regimens at different treatment stages in cancer patients [8].
Discussion
Experience of azoximer bromide use in oncology practice
During experimental, non clinical studies the anticancer activity of AB was studied using three established models: Lewis lung carcinoma, pleural mesothelioma, and at spontaneous carcinogenesis. The first is an experimental model of Lewis lung carcinoma. To simulate Lewis lung carcinoma, 0.5 ml of cancer cell suspension from Lewis lung carcinoma was injected subcutaneously in the back area in 20 C57BL/6 mice weighing 16 g. Azoximer bromide administration was initiated at 48 hours, subcutaneously at a dose of 2.5 mg/kg daily for 5 days. In the second series of experiments, inoculated rat pleural mesothelioma was used. Rats were injected subcutaneously with 0.5 ml of homogenate from the tumor pre-induced by chrysotile asbestos dust. AB was administered 48 hours later using various regimens (5 intraperitoneal or subcutaneous injections of 25 mg/kg once weekly, or 10 intraperitoneal injections of 5 mg/kg 2 times weekly). It is shown that at the same doses (25 mg/kg) and administration frequency (once weekly), a slightly better effect was achieved after subcutaneous injection compared to intraperitoneal administration. The anticancer effect of AB was increased at repeated administration of the drug product (2 times weekly) even when using a lower dose of 5 mg/kg. The third series of experiments was carried out on a model of spontaneous carcinogenesis. AB was administered subcutaneously to mice once weekly at a dose of 25 mg/kg for 40 weeks. Control animals were injected with saline according to the same scheme.
During these experimental studies anticancer effect of AB was observed, but it was less pronounced in case of a more aggressive tumor (Lewis carcinoma). For tumors with lower malignancy (inoculated rat pleural mesothelioma, spontaneous hemoblastoses in mice), more apparent positive results were obtained. It should be noted that azoximer bromide administration in presence of inoculated tumor in no case has led to increased neoplastic process [9]. The initial experience of AB use in oncology practice was aimed to improve tolerance of chemotherapy and major surgical interventions and improve the quality of life of oncology patients. The key clinical observations on the AB use in oncological practice are summarized in Table 1.
| No. | Nosology | Scheme/dose | Number of patients | Result |
|---|---|---|---|---|
| 1 | Colon cancer, III-IV stages | Adjuvant mode, double-blind, placebo-controlled study group 1 mg-6 mg group 2 mg-12 mg group 3 mg-12 mg + 5FU | 49 | Normalization of immunity indicators |
| 2 | Langerhans cell histiocytosis | CT+AB 6 mg (children under 6 years old-3 mg), for 2 weeks 3 times a week, then for 6 weeks 2 times a week | 16 | Normalization of immunity indicators, reduction of tumor volume by 20%-30% |
| 3 | Hodgkin's lymphoma | PCT+AB 6 mg (children under 6 years old-3 mg), for 2 weeks 3 times a week, 2 courses | 12 | Normalization of immunity indicators, reduction in the size of tumor lymph nodes by 30%-60% |
| 4 | Skin melanoma | Adjuvant 6 mg IM every other day for 3 months | 49 | 2-fold decrease in the frequency of cytogenetic disorders, 8-fold increase in 3-year survival |
| 5 | Solid tumors in children (ES, NB, rhabdomyosarcoma, germ cell tumors) | CT+AB subcutaneously 0.15 mg/kg body weight for 5 days | 20 | Normalization of immunity indicators, a decrease in the incidence of infectious complications |
| 6 | Advanced lung cancer | CT+AB | 129 | Significant increased overall survival (p=0.016) and a decrease in the incidence of infectious complications |
| 7 | Localized lung cancer | AB monotherapy, adjuvant regimen | 56 | Significant reduction in metastasis rate of (p=0.017) and normalization of immunity indicators |
| 8 | Kidney cancer | AB monotherapy, adjuvant regimen | 72 | Normalization of immunity indicators |
| 9 | Chronic lymphocytic leukemia | CT+AB or AB monotherapy | 21 | Increasing the efficiency of standard chemotherapy |
| 10 | Breast cancer | 6 mg, IM-9 times between courses of chemotherapy in an adjuvant mode | 94 | Improving the tolerability of chemotherapy (according to NCI-CTC v3.0), improving the quality of life (FACT-G questionnaires), stabilizing immunity indicators, stimulating leukopoiesis |
| 11 | Breast cancer (Т12N0M0) | 6 mg IM, every other day-10 injections | 96 | Normalization of humoral and cellular immunity |
| 12 | Breast cancer | Neoadjuvant mode, 12 mg every other day, 5 injections total | 20 | Changes in the composition of intratumoral lymphocytes, in 30% of cases, therapeutic pathomorphosis of varying degree was noted |
| 13 | Breast cancer | Neoadjuvant mode, 12 mg every other day, 5 injections total | 75 | Therapeutic pathomorphosis of varying degree in the primary tumor and metastatic lymph nodes |
Table 1: Clinical studies of AB use in oncological practice.
One of the first clinical studies of the drug product was a double blind placebo controlled study of AB use in adjuvant mode in combination with 5-fluorouracil in patients with stage III-IV colon cancer who underwent radical or palliative surgical treatment. Three groups of patients were formed. The first experimental group-PO at a dose of 6 mg (once daily, 5 administrations every day) vs. placebo+5 FU simultaneously 2-3 courses, second group- PO 12 mg (once daily, 5 administrations every other day) vs. placebo+5 FU simultaneously and the third group-5 FU 2-3 courses, then PO at a dose of 12 mg (once weekly, 5 administrations) vs. placebo. Subjectively, 100% of patients in the experimental groups reported positive shift in well being, compared to 42.9% in the control group (49 patients). In the experimental groups, positive changes in immunological indicators were observed in 87.2% of patients. The most significant change was the increase of both the relative and absolute peripheral white blood cell counts of CD3+, CD4+ and CD8+ phenotype. In contrast, 90.9% of patients in the placebo group demonstrated negative changes of immunological indicators over time. There were no cases of system or local adverse or allergic reactions associated with AB administration. Various therapy regimens allowed to conclude that azoximer bromide administration in a course dose of 30 mg may be sufficient to maintain initially normal immune parameters. However, in patients with initially low immune parameters associated with chemotherapy conducted a course dose of at least 60 mg is recommended.
During the assessment of the possibility of using AB as supplementary therapy in children with solid malignancies, it was shown that AB use in polychemotherapy contributes to significant reduction in the frequency of infectious bacterial and viral complications, compared with children who received only systemic polychemotherapy (1.8% and 12.5% vs. 6.5% and 24.3%, respectively) (p<0.05). In addition, AB use in conjunction with chemotherapy may reduce the severity of immunosuppression and achieve a significant improvement in immunological indicators, namely, the T-cell component and the phagocytic component (neutrophil stimulation index) (p<0.05). And at the stage of induction therapy, it slightly increases the frequency of responses. There is a positive experience of clinical AB use in children with Hodgkin's lymphoma and histiocytosis. A total of 28 children aged 6 months to 15 years with stage I-II-III-IV Hodgkin's lymphoma (16 patients) and Langerhans cell histiocytosis (12 patients) were evaluated. 7 patients with Langerhans cell histiocytosis were diagnosed with congenital disseminated forms of the disease.
All enrolled patients received AB daily by intramuscular injection before treatment for 5 days at a dose of 3 mg/day (for patients up to 5 years) and at a dose of 6 mg/day (for patients from 5 to 15 years). Further, patients with Hodgkin's lymphoma (16 subjects) were prescribed AB 3 times weekly for 2 weeks, 2 courses in total. Patients with Langerhans cell histiocytosis (11 subjects) were prescribed AB in combination with chemotherapy according to the following scheme: 3 times weekly for the first 2 weeks, then 2 times weekly for 6 weeks. One patient with localized form of histiocytosis received AB (without chemotherapy) according to the scheme: 3 times weekly for the first 2 weeks, then 2 times weekly for the next 6 weeks. Then he received 6 mg once weekly for 10 months (within 1 year he received 65 doses (390 mg) of AB in total).
Increased T-cell counts, neutrophil activity and bactericidal activity and activation of humoral immunity were observed after administration of 5 doses of AB in children with congenital form of Langerhans cell histiocytosis. AB administration in children with Hodgkin's lymphoma correlated with decreased Tkiller counts, partial normalization of the ratio of immunoregulatory cells, activation of humoral immunity indicators and increased phagocytic activity. A positive anticancer effect was reported in association with drug product administration. Clinical presentation was characterized by decreased size of tumor masses by 20%-30% compared to the original size [10].
AB administration during the 1st course of chemotherapy was also associated with regression of tumor peripheral lymph nodes by 30%-60% compared to the original sizes. At the same time, in 5 patients with mixed cell Hodgkin's lymphoma variant out of 7 enrolled in the study complete regression of tumor nodes was observed, and in 2 patients with nodular sclerosis slow regression of tumor nodes was noted. Complete regression of soft tissue masses was observed in 6 patients with histiocytosis during combined 5-day AB+CT therapy and 1 patient with localized histiocytosis after IM administration of AB at a total dose of 180 mg demonstrated complete clinical effect. Azoximer bromide effects in the complex treatment of patients with chronic lymphocytic leukemia were evaluated. The study examined the efficacy of AB addition to the chemotherapeutic agents cyclophosphane and chlorbutin in patients with stage IIIII B-cell lymphocytic leukemia aged 39-84 years, as well as AB use as monotherapy in patients with stage I chronic lymphocytic leukemia. Positive changes in CD3+ CD4+ T-cells, NK cell counts, increased phagocytic activity of neutrophils and immunoglobulin levels over time were noted under the effect of AB. AB contributed to increased chemotherapy efficacy, as well as antibacterial therapy in the development of infectious and inflammatory complications. The clinical and immunological effect of AB was maintained for 140 days.
A number of studies have examined the effect of azoximer bromide in lung cancer. In particular, in advanced lung cancer, the addition of AB to standard chemotherapy resulted in significantly increased overall survival of patients. Thus, significantly increased overall survival (p=0.0164) was reported in a subgroup of patients who had each course of chemotherapy accompanied with AB administration. In addition, these patients had lower number of chemotherapy complications, compared to the subgroup of patients where PO was used only during the last 5 courses of chemotherapy, as well as the reference group.
Complete course of anticancer therapy was received by 75.8% of patients in the subgroup 8 courses (AB+CT), 63.5% of patients in the subgroup 3courses CT+5courses (AB+CT), and 58.5% of patients in the CT group. The authors concluded that PO use together with chemotherapy in patients with advanced lung cancer, in addition to increasing overall survival due to its own anticancer effect, also leads to reduced frequency and severity of infectious complications. In patients with localized lung cancer (stage I-II), adjuvant AB monotherapy after surgical treatment resulted in significant reduction of metastasis frequency 11.2% in the AB group versus 28.8% in the reference group (p=0.017). In addition, patients in the AB group showed signs of activation of specific T-cell and non specific cell mediated (neutrophilic phagocytes and NK cells) immunity. Also, surgical treatment of oncological diseases was associated with changed immune system indicators in the AB group of patients with kidney cancer after nephrectomy. Significantly increase of CD3+ counts by 13.8% and CD4+ counts by 26.2% was demonstrated. The (CD4+/CD8+) ratio was significantly increased by 2 times due to increased T helper counts, also number of NK cells was significantly increased (by an average of 24%). In the large scientific study on the immunoreactivity of cancer patients and the principles of immunocorrective therapy at time of cancer surgical treatment, changes in the immune system indicators were shown in kidney cancer patients and in localized lung cancer patients. Thus, in the AB group, in patients with kidney cancer after nephrectomy significantly increase of CD3+ counts by 13.8%, CD4+ counts by 26.2% was revealed. The (CD4+/ CD8+) ratio significantly increased by 2 times due to increased T-helper counts, also number of NK cells was markedly increased by 24% (p<0.03). In patients with lung cancer, signs of specific T-cell and non-specific cell mediated (neutrophilic phagocytes and NK cells) immunity activation were observed in association with AB administration. Two studies were conducted to assess the effect of azoximer bromide on the postoperative chemotherapy or chemoradiotherapy tolerability in patients with Breast Cancer (BC).
The first study included 94 patients with BC after radical surgical treatment, who received FAC chemotherapy and some of them radiation therapy. Group 1 (standard treatment and AB) included 31 patients. Group 2 (standard treatment without immunotropic therapy)-31 patients. Group 3 (standard treatment+Licopid)-15 patients. Group 4 (standard treatment, licopid and AB)-17 patients. AB was administered intramuscularly 6 mg in a course of 9 injections at days 3 to 19, between courses of chemotherapy. The impact of immunotherapy on the tolerability of adjuvant polychemotherapy was assessed using the NCI-CTC v3.0 criteria, as well as the percentage and quantitative ratio of the incidence of infectious complications. To assess the quality of life, the FACT-G questionnaire was used. The study showed favorable tolerability of the drug product and the absence of adverse events. For immunity parameters, increased activation antigens such as ICAM-3 (CD50), CD38, CD95 and CD11b on blood lymphocytes, compared to control. Under the effect of the drug product, there was an absolute increase in the number of mature B-cells, CD45 RA+ cells and T-helper cells. When evaluating the effect of the drug product on leukopoiesis, it was found that the latter is most likely stimulated by the induction of the synthesis of pro inflammatory Th1-dependent cytokines, which are subsequently required to induce the production of colony stimulating factors that directly stimulate leucopoiesis [11]. Treatment efficacy was confirmed by significant reduction in the chemotherapy toxicity reflected by decreased degree of neutropenia, and reduced frequency of infectious complications by 75% in the azoximer bromide group.
In two groups of patients with breast cancer, quality of life parameters were evaluated using the special FACT-G questionnaire with supplemented questions for toxicity assessment. Patients individually completed questionnaires 4 times before each chemotherapy course. The treatment group included 30 patients; the control group included 29 patients. Patients of the treatment group received AB 6 mg by intramuscular injections in the intervals between chemotherapy courses on the days 3, 5, 7, 9, 11, 13, 15, 17 and 19 of each cycle. In AB group there was a small but consistent increase in general performance status: Physical (by 1 point) and emotional (by 3 points), as well as a decrease in the social domain indicator (by 1 point). In the control group, the quality of life in the domains of social and emotional well being was unchanged, while the indicators of physical performance decreased by 1.5 points. The parameter of ADD toxic manifestations increased by 1 point, while in the control group it decreased by 4 points (the difference is significant, p ≤ 0.05). The average utility indicator that evaluates the change in the total quality of life in the treatment group has a positive value equal to 3, while in the control group a negative value (-3), which indicates a positive impact of AB administration between courses of therapy on the quality of life of patients with breast cancer. Data on physical activity status were also significantly higher in the treatment group. 100% of patients treated with AB completed the planned treatment, in the control group only 83.9% of patients completed the planned 4 courses of chemotherapy on time (p<0.05). The reasons for the interruption of courses of therapy included poor tolerability, persistent leukopenia and pneumonia in one case. In the control group, frequency of various complications was also significantly higher. The proportion of infectious complications was 19.4% (acute bronchitis, pneumonia, acute respiratory viral infections, suppuration of the postoperative scar). In the AB group infectious complications were not reported (p<0.05). The effect of AB on the tolerance of postoperative chemotherapy or chemoradiotherapy in 59 patients with BC was studied. The study has shown that azoximer bromide administration in the intervals between FAC courses improves treatment tolerability and significantly reduces the frequency of adverse effects. Taking into account changes of peripheral blood immunological parameters over time, positive immunomodulatory effect of the drug product was observed, especially in patients with reduced cell-mediated immunity at baseline.
A well marked anticancer effect of AB administered alone as adjuvant therapy was demonstrated in patients with melanoma. This study included 70 patients with morphologically verified stages I-III skin melanoma. In all patients at baseline cytogenetic examination revealed 8% to 40% of lymphocytes with various cytogenetic disorders. Group 1 included 30 patients, who received surgical treatment only. Group 2 included 40 patients who received AB immunotherapy after surgical intervention by 3-month courses of 6 mg-12 mg, administered intramuscularly every other day, depending on their body weight, as well as the results of previous cytogenetic study. Using cytogenetic and clinical criteria, high efficacy of AB as adjuvant therapy after surgical treatment in patients with skin melanoma has been established. The 3-year survival of patients with skin melanoma in surgical treatment only group was 13.3%, while in the AB+ group-92.5%. In addition, AB treatment was correlated with lower probability of disease relapsing (93.3% in the control group versus 47.5% in the AB+ group).
The effect of azoximer bromide on the indicators of cellmediated and humoral components of immune system in the nodular form of BC at the early stages of neoplasia development was demonstrated. The study involved 96 patients with nodular BC (stage T1-2N0M0). Normalization of humoral immunity indicators (Ig A and M levels) in patients of group AB was more pronounced compared to the indicators in patients in standard therapy group. AB use in traditional complex therapy of breast cancer at the initial stages of the disease leads to a pronounced positive change of the immune status, which is demonstrated by normalization of not only cell mediated indicators, but also humoral component of immune system. Considering changing trends in the treatment of cancer patients and the increasingly frequent use of CT in neoadjuvant mode in a number of cancers, the possibility of AB use in the preoperative period in patients with BC was studied.
The purpose of the study was to evaluate the effect of AB on the tumor microenvironment. The study included 20 patients with breast cancer who underwent tumor core biopsy, subsequent AB treatment, and radical surgery. AB was administered intramuscularly at a dose of 12 mg on days 1, 2, 3, 5, and 7 (day 1-the day of the core biopsy). The surgery was performed on the next day after last AB administration. The authors noted the excellent tolerance of the drug product, as well as the absence of complications and side effects. None of the patients had infectious diseases during immunomodulatory therapy and in the postoperative period. IHC test of intratumoral lymphocytes in the core biopsy material and the tumor operating material showed that azoximer bromide is characterized by an immunomodulatory effect on intratumoral cells. Decreased CD4+ cell counts and T-cytotoxic (CD8+) lymphocyte counts were observed if their initial levels were above the median, and increased counts were seen their initial levels were below the median (the median for CD8+ cell count is 41%, for CD4+ cell count 47%). Additionally, this study demonstrated that the drug product has a positive effect on the tumor (pathomorphosis induction), associated with a subpopulation of intratumoral CD4+ lymphocytes and decreased CD8/CD4 index. According to the results of histological examination of a surgically removed tumor after AB immunotherapy, 30% of patients had therapeutic pathomorphosis (graded by lavnikova scale). Thus 3 patients had grade I (>50% of the tumor parenchyma preserved), 2 patients had grade II (20%-50% of the tumor parenchyma reserved), and 1 patient had a complete absence of tumor parenchyma in the removed breast tissue (grade IV pathomorphosis).
The case of therapeutic pathomorphosis aroused great interest and many questions from clinicians and pathomorphologists, so the authors conducted a detailed evaluation of this case. Morpho immunehistochemical characteristics of the material obtained during core-biopsy (before treatment) and the surgical material were compared. This case was discussed in detail at the International conference haematopoiesis immunology in 2015 (Suzdal), and a detailed morphological and immunohistochemical characterization of the cell composition of inflammatory infiltrate in the tumor bed of breast cancer with complete therapeutic pathomorphosis after AB immunotherapy was published in 2015. The results of this pathomorphological analysis showed that the inflammatory infiltrate was represented by both non-specific and specific immunity cells, with an equal content of cell-mediated and humoral immunity elements. Macrophage elements (CD68+) were represented by both discretely arranged histiocytes and epithelioid macrophages forming granulomas without necrosis and separate accumulations of giant multinucleate cells of foreign bodies. About 15%-20% of cells in the infiltrate were represented by granulocytes with a predominance of neutrophils. It was found that the primary tumor contains a large number of discretely located tumor-associated M2-type macrophages responsible for anti-inflammatory and reparative processes.
Based on the results of morphological and immunophenotypic analysis of breast tissue changes, a number of non-neoplastic pathological processes that have mammographic, clinical and morphological similarities with invasive cancer were excluded. The revealed immunomorphological changes were deemed to be a combination picture of specific immune response mediated by the presence of T- and B-lymphocytes and plasma cells and acute inflammatory process accompanied by the formation of multiple abscesses and granulomas around necrotic tissue.
Taking into account the unexpected pronounced anticancer effect, larger clinical study was conducted to assess the effect of azoximer bromide use in neoadjuvant mode for treatment of primary operable breast cancer. The study was conducted in 75 patients with BC, 50 patients received AB in neoadjuvant mode (treatment group), 25 patients did not receive AB (control group). AB, lyophilizate for solution for injection and topical use, at a dose of 12 mg was injected intramuscularly once daily on the days 1, 2, 3, 5 and 7 before surgery according to the scheme described by Shamilov FА. No side effects were reported during the treatment course. On day 8, patients of both groups underwent radical mastectomy followed by histological examination of the surgical material and determination of the degree of tumor therapeutic pathomorphosis according to lavnikova scale. Evaluation of the primary tumor has shown therapeutic pathomorphosis of various degree in 64% of patients. In most cases (58% of all patients), grade I pathomorphosis was noted, and in 3 (6%) patients grade II pathomorphosis was observed. Pathomorphosis in metastatic lymph nodes was observed less frequently than in the primary tumor in 22.7%. Pathomorphosis severity of metastatic tumor cells in the lymph nodes was also lower than in the primary tumor-in all cases, degree I therapeutic pathomorphosis was noted. In patients from the control group who did not receive AB, tumor tissue pathomorphosis was not observed.
All the clinical observations and studies described above show favorable tolerability and high safety of AB in cancer patients. In addition, it should be noted that azoximer bromide safety was confirmed in a post-marketing study conducted in the EU (Slovakia). The study included 500 patients from 15 clinical sites.
Based on the study results, the most optimal schemes were proposed for using azoximer bromide both in adjuvant mode in patients with GI cancers, kidney cancer, lung cancer, melanoma and breast cancer and as neoadjuvant therapy for primary operable breast cancer.
Conclusion
This literature review analyzed novel mechanisms that mediate the direct anticancer effect of the azoximer bromide. A significant number of clinical observations and research papers on the use of azoximer bromide in cancer patients together with favorable safety profile allows us to consider AB as a promising anticancer agent for use both as part of complex/combination therapy as well as monotherapy.
Ethics Approval and Consent to Participate
All studies described had a procedure of consent signing by patient.
Consent for Publication
Authors provide consent for publication.
Availability of Data and Materials
All studies results mentioned are available in the open courses, as well as open reports of scientific research.
Competing Interests
None.
Funding
None.
Authors’ Contributions
Grivtsova-article design, data analysis, writing, Falaleevacorrection, Tupitsyn-editorial revision.
Acknowledgements
L Yu Grivtsova, NA Falaleeva and NN Tupitsyn wrote and designed this review.
References
- Sheikh Sajjadieh M, Edalati M, Ajami A, Sheikh Sajjadieh E. The effect of azoximer bromide in treatment irritable bowel syndrome. Allergy. 2017;72:758-823.
- Kostinov MP, Latysheva EA, Kostinova AM, Akhmatova NK, Latysheva TV, Vlasenko AE, et al. Immunogenicity and safety of the quadrivalent adjuvant subunit influenza vaccine in seropositive and seronegative healthy people and patients with common variable immunodeficiency. Vaccines. 2020;8(4):639-640.
[Crossref] [Google Scholar] [PubMed]
- Bazdyrev E, Rusina P, Panova M, Novikov F, Grishagin I, Nebolsin V, et al. Lung fibrosis after COVID-19: Treatment prospects. Pharmaceu. 2021;14(8):806-807.
[Crossref] [Google Scholar] [PubMed]
- Kostinova AM, Yukhacheva DV, Akhmatova EA, Akhmatova NK, Kostinov MP, Stolpnikova VN, et al. The effect of influenza vaccines on maturation of dendritic cells generated from bone marrow. Austin J Vacc Immunother. 2021;5(1):1011-1012.
- Shakya AK, Nandakumar KS. Applications of polymeric adjuvants in studying autoimmune responses and vaccination against infectious diseases. J R Soc Interf. 2013;10(79):20120536.
[Crossref] [Google Scholar] [PubMed]
- Kostinov MP, Markelova EV, Svitich OA, Polishchuk VB. Immune mechanisms of SARS-CoV-2 and potential drugs for the prevention and treatment of COVID-19. Pulmonol. 2020;30(5):700-708.
- Tobias A, Galan I, Banegas JR. Non‐linear short term effects of airborne pollen levels with allergenic capacity on asthma emergency room admissions in Madrid, Spain. Clin Exp Alle. 2004;34(6):871-878.
[Crossref] [Google Scholar] [PubMed]
- Fernandez-Gonzalez M, Ribeiro H, Pereira JR, Rodriguez-Rajo FJ, Abreu I. Assessment of the potential real pollen related allergenic load on the atmosphere of Porto city. Sci Total Env. 2019;668:333-341.
[Crossref] [Google Scholar] [PubMed]
- Cabrera M, Subiza J, Fernandez-Caldas E, Garzon Garcia B, Moreno-Grau S, Subiza JL, et al. Influence of environmental drivers on allergy to pollen grains in a case study in Spain (Madrid): Meteorological factors, pollutants and airborne concentration of aeroallergens. Env Sci Pollu Res. 2021;28(38):53614-53628.
[Crossref] [Google Scholar] [PubMed]
- Stsepetova J, Baranova J, Simm J, Parm U, Roop T, Sokmann S, et al. The complex microbiome from native semen to embryo culture environment in human in vitro fertilization procedure. Reprod Biol Endocrinol. 2020;18:1-3.
[Crossref] [Google Scholar] [PubMed]
- Hou D, Zhou X, Zhong X, Settles ML, Herring J, Wang L, et al. Microbiota of the seminal fluid from healthy and infertile men. Fert Ster. 2013;100(5):1261-1269.
[Crossref] [Google Scholar] [PubMed]
Citation: Grivtsova LY, Falaleeva NA, Tupitsyn NN (2023) Azoximer Bromide as an Activator of Cellular Immunity: New Opportunities for Practical Use in Cancer Patients. J Stem Cell Res Ther. 13:579.
Copyright: © 2023 Grivtsova LY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

