Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2019) Volume 10, Issue 2
Availability and Status of Vaccine Cold Chain Equipment in Cameroon
Saidu Yauba1*, Ename E Harmelle2, Vouking Zambou Marius2, Nkwain Jude1, Kamga Delphine1, Tonga Calvin2, Bayiha Christain2, Ewane Leonard2, Biloa Alain3, Mbollo Marianne4, Mbu Robinson5, Dicko Hamadou6 and Nzuobontane Divine12Expanded Program on Immunization, Ministry of Public Health Cameroon, Cameroon
3World Health Organization, Country Office Cameroon, Yaounde, Cameroon
4United Nation’s International Children’s Emergency Funds, Ministry of Public Health Cameroon, Country Office, Yaounde, Cameroon
5Directorate of Family Health, Ministry of Public Health Cameroon, Cameroon
6Gavi, The Vaccine Allaince, Geneva, Switzerland
Received: 20-Feb-2019 Published: 20-Mar-2019
Abstract
Background: Availability of adequate number of optimal cold chain equipment is indispensable for expanded programs on immunization. Lack of this equipment can hinder a country’s progress towards its immunization coverage and equity goals. In this study, we evaluated the availability and status of vaccine cold chain equipment in Cameroon so as to facilitate planning for repairs, maintenance, replacement and expansion of cold chain infrastructure.
Methods: This cross-sectional study was conducted in Cameroon, a country that has witnessed a sizable decline in its immunization coverage in recent years. Pre-tested and validated questionnaires were used to collect data from districts and health facilities. Completed questionnaires were double entered into Census and Survey Processing System and analyzed using SPSS, version 22.
Results: Overall, 4,568 sites were surveyed, including 189 health districts and 4,379 health facilities. Of the 189 surveyed districts, 75% were equipped with WHO-prequalified vaccine refrigerators, 7% were unequipped with any refrigerator, while 3% were equipped with broken refrigerators. In addition, 12% were equipped with absorption (PIS) refrigerators and the remaining 3% with domestic refrigerators. Of the 4,379 surveyed facilities, 38% were unequipped with any refrigerator and 14% were equipped with broken refrigerators. Only 2% of facilities were equipped with WHO-prequalified refrigerators. The remaining facilities met their cold chain needs with absorption (28%) and domestic (18%) refrigerators. With regards to storage capacity gaps, 45% of health districts had capacity gaps in 2017, a percentage that is projected to reach 75% by 2021. Unlike districts, almost all facilities had cold chain capacity gaps in 2017 and this percentage is expected to reach 99% by 2021 if no intervention is implemented.
Conclusion: Our findings suggest that the cold chain system in Cameroon, particularly at facility level is severely compromised. This challenge highlights the need for urgent interventions to restore adequate and functional cold chain capacity at these levels as this will be necessary to boast immunization coverage and equity.
Keywords
Immunization; Cold chain equipment; Cold chain capacity gaps; Coverage and equity; Districts and health facilities; Vaccine refrigerators
Introduction
The Expanded Program on Immunization (EPI) has made tremendous progress in addressing the burden of vaccine preventable diseases, which claimed millions of lives in the pre-EPI era [1]. At inception in 1974, global coverage for the third dose of Diphtheria, Tetanus and Pertussis containing vaccine (DTP-3) was low, hovering around 5%. But since then, coverage for this antigen has risen steadily, surpassing 85% in 2010 [2]. This progress, however, has now stagnated as global coverage for this antigen has been oscillating between 84% and 85% since 2010 [3]. This stagnation is more prominent in the World Health Organization (WHO) African region, where coverage levels have not only remained constant, but are at risk of slipping away in many countries [4]. In Cameroon for instance, DTP-3 coverage dropped from 89% in 2013 to 86% in 2017 [5]. This negative trend also extends to other key antigens, including BCG, oral polio vaccine and the measles combined vaccine amongst others [6]. Apparently, these failings put Cameroon’s progress towards the goal of reaching 90% coverage nationally by 2020 as outlined in the 2020 Global Vaccine Action Plan, largely off-track [7].
A number of reports have attempted to identify potential reasons for these failings [8-11] . Amongst the identified reasons, weaknesses in the cold chain system appear to be the most prominent bottleneck that is hindering the program’s progress towards its coverage and equity goals. The system has become increasingly fragile in recent years and is characterized by diverse problems, including insufficient cold chain storage capacity at all levels, heavy use of obsolete and domestic refrigerators, weak temperature monitoring and control systems, and high rates of cold chain equipment (CCE) failure, amongst others. These bottlenecks pose a major economic and public health risk to the program. For instance, in June 2015, a dysfunctional cold room damaged roughly 500,000 doses of inactivated polio vaccine one month prior to its introduction [12] and this incident created a generalized stock out of this vaccine, which in turn impacted its coverage [13]. Similar observations were noted with the bivalent oral polio vaccine, where the quality of over 800.000 doses of this antigen were compromised as a result of prolonged heat exposure at operational level [14].
Given this recurrent and consequential EPI logistic crises, coupled with the need to introduce new vaccines, it was important to assess the status of the vaccine cold chain system in Cameroon. In this paper, we present the findings of an assessment that was designed to evaluate the availability and status of CCE in Cameroon so as to facilitate planning for repairs, maintenance, replacement and expansion of cold chain infrastructure.
Methodology
Study setting
The study was conducted in Cameroon, a lower-middle-income country with a population of nearly 25 million people [15]. The country lies between latitudes 1° and 13° N and longitudes 8° and 17° E and has a total surface area of 475,442 Km2 [16]. Located in Central Africa, Cameroon shares a border with Chad, Central African Republic, Equatorial Guinea, Gabon Nigeria and the Republic of Congo, countries which are known to have very fragile immunization systems [17].
Cameroon’s immunization system has grown progressively ever since it became fully established in 1982. At inception, the program was offering six pediatric vaccines. This number rose to 12 antigens by 2017, and there are plans in place to introduce three additional vaccines by 2021 [6]. The birth cohort has also tripled during this period, rising from 0.3 Million infants in the early 1980s to about 0.9 Million in 2017 [15]. This dramatic evolution has put enormous pressure on the cold chain infrastructure, which until now is yet to undergo a corresponding expansion to accommodate the continually rising cold chain capacity needs. This constraint has on some occasions let to a substantial loss of vaccine stock [12]. Similarly, a recent temperature monitoring study showed that a significant proportion of vaccines are exposed to damaging temperatures at district and facility levels [18].
Study Procedures
Study tools
The WHO Immunization Logistic Assets Inventory Management Tool was adapted to align with Cameroon’s cold chain system [19]. In addition, the tool was slightly modified to meet requirements of the Gavi Cold Chain Equipment Optimization Platform (CCEOP) -a US $250 Million funding scheme that was established in 2015 to support Gavi-eligible countries transform their cold chain systems [20]. The final tool, which was pretested and validated by the logistic working group, contained questions on health facility/district background (including name, location, type, status, target population, access to electricity and immunization service delivery amongst others) and characteristics of CCEs (e.g., equipment type, manufacturer, model, capacity, age, energy source, functional status and availability of voltage regulators and temperature monitoring devices amongst others).
Trainings
Following written approvals from Ministry of Public Health [21], data collectors were recruited and trained on all aspects of the study, including the completion of the data collection tools. The trainings were complimented with practical exercises, which enabled study staff to simulate the data collection process. Trained staff were then deployed to the field according to an approved data collection plan.
Data collection process
Upon arrival at each site, the staff obtained and documented background information of the site on the appropriate section of the questionnaire. They then inspected the available CCE, during which they abstracted and documented the characteristics of the equipment. At the end, the staff reviewed the completed questionnaire for accuracy and completeness and ensured that all inappropriate entries were corrected before signing and dating the questionnaire. The site representative also signed, dated and stamped the questionnaire, attesting that the information recorded was accurate, consistent with the sources and verifiable. To guarantee data quality, central and regional supervisors performed random spot checks on 10% of sites.
Data management and analysis
Completed questionnaires were reviewed for accuracy and completeness by trained supervisors and any identified issues were resolved before data entry. Reviewed questionnaires were double entered into Census and Survey Processing System (CSPRO, version 7.1.2) by trained data entry clerks. The data were then processed and analyzed using SPSS version 22 and Microsoft Excel 2016 version according to a written statistical analysis plan. Summary statistics were used to estimate the frequency and percentages of the variables of interest. Furthermore, capacity gaps were estimated by deducting the available capacity from the required cold chain capacity at district and facility levels. Non-vaccinating health facilities (n=629) were included in the gap analysis because we anticipate that, in a bid to expand immunization coverage and equity, these facilities may start delivering immunization services before the end of the planning period (2017-2021). As such, the gap analysis was based on a total of 189 districts and 4,179 functional health facilities.
Results
Baseline characteristics
A total of 4,568 sites were surveyed, including 189 health districts and 4,379 health facilities. All districts were functional at the time of the survey as opposed to 95% (4179/4,379) of health facilities. The 200 non-functional facilities were excluded from the final analysis. Table 1 presents selected baseline characteristics of the surveyed sites. As illustrated, all surveyed districts and 57% of health facilities were publicly owned. Of the 4,179 surveyed facilities, 47% were integrated health centers, 32% were health centers and 7% were hospitals.
| Characteristics of surveyed sites | District | Health Facility |
|---|---|---|
| Surveyed site is functional | ||
| Yes | 100 (189) | 95(4,179) |
| No | 0(0) | 5 (200) |
| Status of Functional sites, % (n) | ||
| Public | 100 (189) | 57 (2,510) |
| Private | 0 (0) | 43 (1,869) |
| Access to electricity, % (n) | ||
| Sites has access to electricity, | 88 (166) | 70 (2,912) |
| Sites receives at least 8 hours of electricity per day | 80 (151) | 62 (2,590) |
| Access to a telephone Network, % (n) | ||
| Yes | 99 (187) | 86 (3,590) |
| No | 1 (2) | 14 (671) |
| Types of immunization service dispensed by site % (n) | ||
| Fixed-post immunization only | NA* | 35 (1,476) |
| Fixed-post immunization and outreaches | NA | 50 (2,072) |
| None | NA | 15 (631) |
| Site has at least one trained staff on vaccine management, % (n) | ||
| Yes | 5 (10) | 10 (431) |
| No | 95 (179) | 90 (3,748) |
*NA, not applicable
Table 1: Baseline characteristics of surveyed sites.
Regarding access to electricity, 88% (166) of districts were located in zones with electricity network but only 80% (151) had access to more than eight hours of electricity per day. A similar trend was observed with health facilities, but with lower proportions (Table 1). Unlike access to reliable electricity, access to telephone networks appeared to be somewhat better, with 99% of districts and 86% of facilities having access to at least one mobile provider.
All districts provided EPI services, essentially serving as vaccine storage sites and coordinating the implementation of vaccination activities within the district. Eighty-five percent (3,548) of facilities dispensed EPI services, either via fixed-post immunizations (35%) or a combination of fixed-post immunizations and outreaches (50%). Despite these high percentages, only 5% of district and 10% health facilities had at least one staff, who has been trained on vaccine delivery and cold chain management.
Availability of cold chain equipment at district level
Figure 1 shows the availability of vaccine refrigerators at districts. As illustrated, 75% (141) of districts were equipped with CCE that are currently approved by the WHO Performance, Quality and Safe (PQS) system [22]. In particular, there were three main brands of prequalified vaccine refrigerators at this level, namely Dometic, Vestfrost and Sundanzer. Aside districts with pre-qualified refrigerators, 12% (21) of districts were equipped with old generation equipment, notably Sibirs and Electrolux. Although these brands were previously approved under the WHO Product Information Sheet (PIS) system, these brands are no longer recommended for vaccine storage because of their increased propensity to expose vaccines to damaging temperatures [23]. Similarly, seven districts (3%) were equipped with domestic refrigerators, which like the former, are not recommended for vaccine storage. Surprisingly, there were 13 districts (7%) without any device and the remaining 3% (7) had broken devices. The predominant reasons for broken devices were lack of access to spare parts (22%), competent technicians (21%), bottle gas/kerosene to fuel absorptiontype refrigerators (6%) and funds for repair and maintenance (4%) amongst others.
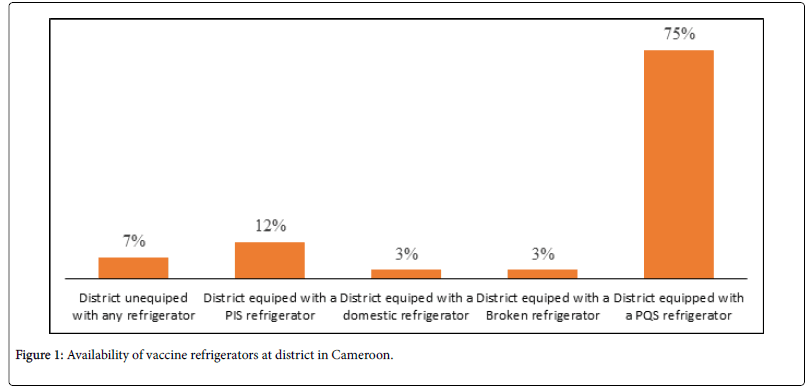
Figure 1. Availability of vaccine refrigerators at district in Cameroon.
Availability of cold chain equipment at health facility level
Figure 2 shows the availability of vaccine refrigerators at health facility level. As shown, 38% (1,579) of health facilities were unequipped with any device. Beside this challenge, a significant proportion of facilities met their CCE needs with PIS (28%) and domestic refrigerators (18%). In 14% (585) of facilities, available CCE were faulty and the reasons for this were similar to those noted at the district level. Finally, only 2% (86) of surveyed facilities had refrigerators approved by the current WHO PQS system.
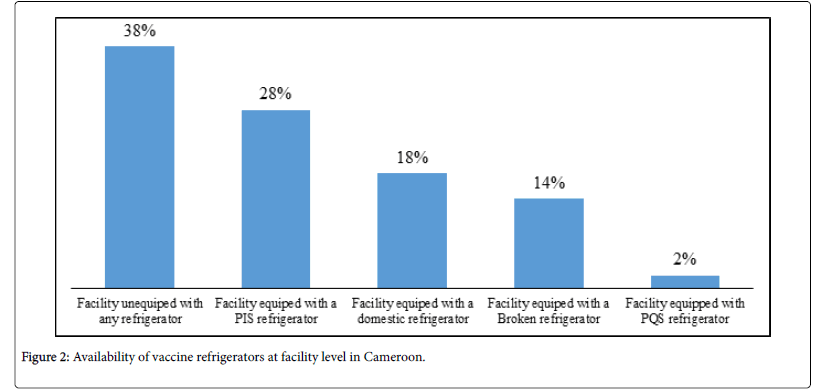
Figure 2. Availability of vaccine refrigerators at facility level in Cameroon.
Distribution of sites with cold chain capacity gaps at district and facility level
Figure 3 below shows the year wise distribution of storage capacity gaps at district and facility levels. Scale of storage capacity gaps at district and facility levels shows the year-wise distribution of capacity gaps at district and facility level. As illustrated, 45% (85) of districts had capacity gaps in 2017, a percentage that is projected to reach 75% (141) by 2021. Unlike districts, almost all facilities (4114/4179) had capacity gaps in 2017 and this percentage is expected to reach 99% (4116) by 2021 if no intervention is implemented. In a majority of health facilities (92%), of capacity gaps can be effectively addressed by providing each of these facilities with a 60L refrigerator. In districts with capacity gaps, the need can be addressed by providing each of these districts with two 60L refrigerators.
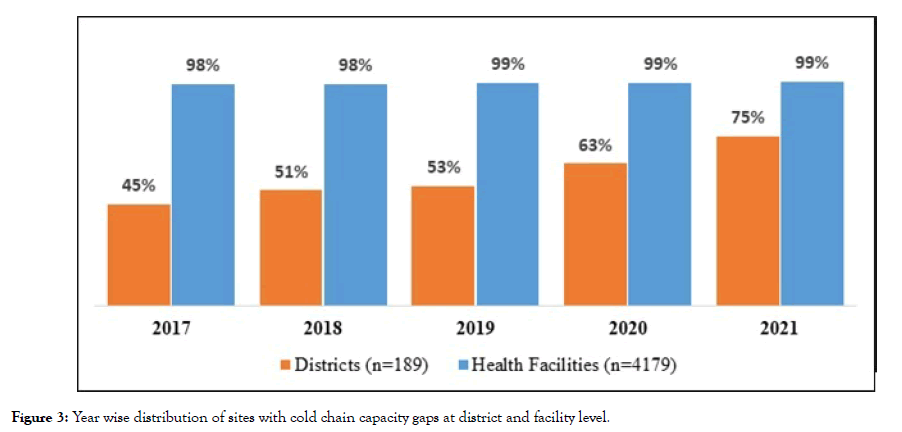
Figure 3: Year wise distribution of sites with cold chain capacity gaps at district and facility level.
Discussions
The overarching goal of this cross-sectional study was to inform the government of Cameroon on its procurement decisions when applying for Gavi Health System Strengthening (HSS) grants and funding from Gavi’s CCEOP. As such, the study was designed to assess the availability and functional status of CCEs in all districts and health facilities in Cameroon, regardless of whether they were offering immunization services or not. We found that only 2% of facilities were equipped with devices that are approved for vaccine storage by the WHO PQS system. This finding, which lends support to an observation by Gavi [24], is of critical concern because lack of functional devices at facility level can disrupt regular conduct of immunization sessions, which in turn, can lead to missed opportunities for vaccination with a resultant effect on coverage and equity goals [25]. Second, the heavy use of non-recommended devices can compromise the quality of vaccines because these devices are known to have an increased potential to expose vaccines to damaging temperatures [18].
The above observation has contributed to the overwhelming capacity gaps observed at district (45%) and facility levels (98%). This capacity constraint has already been associated with several untoward consequences, including hindering the program’s ability to attain its coverage and equity goals, deterring the introduction of new vaccines (notably, human papilloma Vaccine) and creating significant economic losses for the government. To address these consequences, there is a need to leverage the cold chain planning process, which recommends programs to periodically evaluate capacity, estimate needs, develop cold chain plans, select optimal equipment, mobilize resources and execute cold chain plans [25].
Lack of functional devices at facility level has led many facilities to adopt coping mechanisms to sustain coverage levels. According to published reports, these mechanisms involve storing and collecting vaccines from nearby districts or facilities prior to initiating a vaccination session and returning leftovers at the end of each session [26,27]. This strategy, which is apparently costly for the system (both in terms of transport cost and staff time), limits the ability of facilities to conduct daily immunization sessions as recommended by the EPI norms and standards [28]. The strategy also increases the risk of missed opportunities for vaccinating children and clearly cannot sustainably drive improvements in immunization coverage and equity.
Although the availability of optimal CCE appeared to be better at district level, the findings that 7% of districts lacked any device and that 3% were equipped with broken CCE devices are major concerns. This is because these districts serve about 113 facilities and lack of CCE at these depots can disrupt regular vaccine distribution to facilities. Despite this challenge, little is known about how these districts cope with inadequate cold chain infrastructure, highlighting the need for further research into this area.
Our results also suggest that management of CCE appears to be weak at district and facility levels, a weakness that invariably contributes to high proportions of broken equipment at these levels. We found that most staff managing CCE were untrained (90%). This finding, which is in line with previous observations limits staff ability to perform basic routine maintenance tasks such as defrosting refrigerator or cleaning solar panels [18]. This situation is further compounded by lack of reliable access to critical inputs for curative maintenance at these levels, including spare parts, competent technicians and funding. Indeed, immediately after this assessment, the EPI with the support of its partners, implemented a repair campaign with the goal of salvaging 300 broken vaccine refrigerators; however, the campaign was suspended because of difficulties in accessing spare parts (for both Sibir and Dometic refrigerators) locally as well as challenges in accessing competent technicians. These lessons suggest that to ensure continuous CCE functionality at operation levels, there is the need to: 1) ensure proper staff training on routine maintenance tasks; 2) set up a reliable spare parts management system, which can ensure a sustainable access to these rare products; 3) create a pool of competent technicians and other necessary maintenance staff and 4) ensure adequate funding for maintenance activities. Having these systems in place will be critical to avert the failures observed with previous CCE investments. For instance, in 2010, the French government invested over €8 million to procure and deploy Sibir refrigerators but by 2015, over half of these equipment were nonfunctional, thereby limiting the full benefits of the investment to the immunization program.
Another important finding emanating from our study is that 80% of districts and 62% of health facilities had access to reliable electricity supply. This finding has enabled the EPI to segment its districts and facilities so that each one receives a device that is appropriate for its operational context. This understanding led to a clear delineation on sites to be equipped with Ice Line Refrigerators (ILR) and Solar Direct Drives (SDD).
Although this study has provided useful information about the status of cold chain infrastructure in Cameroon, certain limitations must be acknowledged. First, the goal of collecting data from all health facilities could not be attained because of security threats from Boko Haram insurgency in 5% of health districts. As such, the data summarized in this paper may not be entirely representative. Second, data were collected from facilities based on lists provided by regional delegations of public health. Field experience demonstrated that these lists were not up to date as in some cases unlisted facilities were found, particularly in urban cities. This limitation suggests that our data might have missed some health facilities in urban cities. In addition, obtaining accurate target population data for each facility was challenging, as many of them, (particularly those in urban areas) were unaware of their targets. Despite these limitations, we strongly believe that the present study has provided useful information, which can be leveraged to transform the cold chain infrastructure in Cameroon.
Indeed, the findings were leveraged by the EPI and its partners to develop a US$11.3 funding proposal, which was eventually approved by Gavi’s, under its CCEOP scheme. The approved proposal envisioned the procurement, deployment and installation of 1,554 SDD, 1,528 Ice Line refrigerators, 2,681voltage regulators and 3,082 Continuous Temperature Monitoring devices. The goal of the grant is to address existing capacity gaps in 141 districts and 70% (2,841) of health facilities by 2021. Measures have been taken to address capacity gaps in the remaining 15%, where required cold chain capacity was less than 15 litres. According to current plans, these facilities will be provided with cold boxes, which will be procured using HSS funding. HSS funding will also be leveraged to address capacity gaps at central and regional level.
While this progress is commendable, it is important to highlight that addressing cold chain capacity challenges alone may not translate into improvements in coverage and equity goals. There is the need for major investments in other critical components of the immunization system, including strengthen the capacity of human resources, improving data management systems, increasing funding flow and improving repair and maintenance systems amongst others. These parallel investments will be critical to ensuring that the ongoing Gavi investment in CCE yields the greatest benefits to population of Cameroon.
Acknowledgement
The authors gratefully acknowledge the contributions from, Ashvin Ashok, Zongo E Samuel, Hassan Bachir, Charlotte Njua, Fombon Emmanuel, Asana Miranda, Zogo Hirihiri, Carine Olinga, Glenn Muffih and Brahim Oumar Youssouf. This study was nested in a project funded by The Bill and Melinda Gates Foundation. Therefore, we will like to thank the foundation for their financial assistance. The Foundation had no role in the design, conduct, analysis and reporting of this cross-sectional study. In addition, the foundation and as well as the institutions of the authors, including the Clinton Health Access Initiative, EPI, WHO and UNICEF had no role in the decision to submit the article for publication. While the assistance of these institutions has been important, only the authors are responsible for the opinions and interpretations presented.
REFERENCES
- Reid M, Fleck F.The immunization programme that saved millions of lives. Bull World Heal Organ. 2014;92:314-315.
- Center for Disease Control and Prevention. Global Routine Vaccination Coverage, 2010, MMWR Morb Mortal Wkly Rep. 2010;60:1520-1522.
- Feldstein LR, Mariat S, Gacic-Dobo M, Diallo MS, Conklin LM, Aaron S, et al. Global Routine Vaccination Coverage, 2016. Morbidity and Mortality Weekly Report. 2017;66:1252-1255.
- Tarantola D, Hacen M, Lwanga S, Clements CJ. Is Immunization Coverage in Africa Slipping? An Evaluation of Regional Progress to 2013. Ann Vaccines Immunization. 2014;1:1007.
- Gavi. Cameroon DTP3/immunisation coverage. 2017.
- Ministry of Public Health, Cameroon. Mid-term Evaluation of the 2014-2019 Comprehensive Multi-Year Plan. 2018.
- World Health Organization. The Global Vaccine Action Plan. 2013.
- Gavi. Cameroon Program Capacity Assessment. 2017.
- Kwedi NS, Renee C, Nsangou M, Binde M, Nolna TD, Zogo PO. Factors influencing the performance of routine immunization in urban areas: A comparative case study of two cities in Cameroon: Douala and Yaoundé. Vaccine. 2018;36:7549-7555.
- Clinton Health Access Initiative. Baseline assessment report, Yaounde. 2016.
- Clinton Health Access Initiative. Training needs assessment among central, regional and district EPI staff. 2016.
- Scotney S, Snidal S, Saidu Y, Ojumo A, Ngatia A, Bagana M, et al. Succeeding in new vaccine introduction: lessons learned from the introduction of inactivated poliovirus vaccine in Cameroon, Kenya, and Nigeria. J Infect Dis. 2017;216:S130-S136.
- Programme Elargi De Vaccination. Rapport D’activités. 2016.
- Edengue Jean Marie. Note de présentation sur le dysfonctionnement d’une des chambres froides positives du dépôt central du GTC PEV à Yaoundé”. 2015.
- Worldometer. Population of Cameroon. 2018.
- Worldatlast. Location of Cameroon. 2018.
- Wealth Health Organization. Roadmap for implementing the addis declaration on immunization: Advocacy, action and accountability. 2017.
- Yauba S, Joelle S, Jude N, Tracy BO, Marie K, Charles N, et al. Temperature Monitoring in the Vaccine Cold Chain in Cameroon. J Vaccines Vaccin. 2018;9:384.
- Wold Health Organization. Immunization logistic assets inventory management tool. 2018.
- Gavi. Application for cold chain equipment Support. 2016.
- Ministere de la Sante Publique, Cameroon. Decision No D21-110/L/ Minsante/Sg/Dsf/Gtc-Pev/Pevr-Log/Ulog. Lettre de Ministere de la Sante Publique A Mesdames Et Messieurs Les Delegegues Regionaux De La Sante Publique. 2015.
- World Health Organization. The WHO performance, quality and safety system. 2018.
- Robertson J, Franzel L, Maire D. Innovations in cold chain equipment for immunization supply chains. Vaccine. 2017;35:2252-2259.
- Gavi. Cold chain equipment optimization platform. 2016.
- Ashok Ashvin, Brison Michael, Le Tallec Yann. Improving cold chain systems: Challenges and solutions. Vaccine 2017;35:2217-2223.
- Yakum MN, Ateudjieu J, Walter EA, Watcho P. Vaccine storage and cold chain monitoring in the North West region of Cameroon: A cross sectional study. BMC Res Notes. 2015;8:1-7.
- Ateudjieu J, Kenfack B, Nkontchou BW, Demanou M. Program on immunization and cold chain monitoring: the status in eight health districts in Cameroon. BMC Research Notes. 2013;6:101.
- Ministry of Public Health, Cameroon. EPI Norms and Standard. 2018.
Citation: Yauba S, Harmelle EE, Marius VZ, Jude N, Delphine K, Calvin T, et al. (2019) Availability and Status of Vaccine Cold Chain Equipment in Cameroon. J Vaccines Vaccin 10:400. doi: 10. 24105/2157-7560.10.400
Copyright: © 2019 Yauba S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

