Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Mini Review - (2023) Volume 11, Issue 5
Aortic Hiatus and Treatment for Acute Aortic Dissections
Katsuhiko Oda*Received: 18-Sep-2023, Manuscript No. JVMS-23-23081; Editor assigned: 20-Sep-2023, Pre QC No. JVMS-23-23081 (PQ); Reviewed: 10-Oct-2023, QC No. JVMS-23-23081; Revised: 17-Oct-2023, Manuscript No. JVMS-23-23081 (R); Published: 24-Oct-2023, DOI: 10.35248/2329-6925.23.11.539
Abstract
Treatment options did not exist for Acute Aortic Dissection (AAD) until the 1940s. de Bakey, et al. claimed that surgical procedures should be used for all types of AAD categorized according to the de Bakey classification (1965). However, since 1970, the Stanford classification has recommended that surgery should be used for type A, and medical treatment for type B. Currently, Thoracic Endovascular Aortic Repair (TEVAR) is a third option. There is still no consensus on these three treatment options for AAD treatment. Herein, I report a strategy of combining tear-oriented initial surgery and subsequent TEVAR for type A AAD, and its outcomes. I focused on the role of Aortic Hiatus (AH) in the outcome of AAD. Timely and precise TEVAR for closing of patent tears that cause False Lumen Expansion (FLE) above the AH may improve outcomes. This strategy may be useful in type A AAD treatment. I also present an overview of the history of AAD treatment and of the available strategies.
Keywords
Aortic hiatus; Acute aortic dissection; Thoracic endovascular aortic repair; False lumen expansion; Open aortic repair
Abbreviations
AH: Aortic Hiatus; FL: False Lumen; SINE: Stent Graft-Induced New Entry; TEVAR: Thoracic Endovascular Aortic Repair; TL: True Lumen
Introduction
The cardiovascular surgical team at Iwate Prefectural Central Hospital adopted a strategy of combining tear-oriented initial surgery and subsequent TEVAR for type A AAD in 2013 (Figure 1) [1]. It was initially predicted that TEVAR alone would be not able to control the FLE after initial surgery, and that some cases would require thoracoabdominal aortic replacement. This was explained to the patients. After 10 years, thoracoabdominal aortic replacement was not needed because of the adoption of this strategy.

Figure 1: Mt. Iwate and Iwate prefectural central hospital.
The Aortic Hiatus (AH) is the only rigid structure surrounding the distal aorta. This structure may play a pivotal role in AAD outcomes. Here I present an overview of the history of AAD treatment and explain the advantages of our strategy.
Treatment of AAD in the 20th Century
AAD had no treatment options until the 1940s. However, the surgical challenges in AAD repair were successfully overcome with the development of cardiopulmonary bypass, aortography techniques, and prosthetic grafts between 1950 to 1960. de Bakey classification of the outcomes of surgical and medical management of AAD was presented in 1965 (Figure 2) [2]. Originally, surgical treatment was considered better than medical treatment for de Bakey type I, II, and III AAD. The survival percentages after 1 year in operative and non-operative cases were 70% and 7%, respectively. Further, the Stanford classification was presented in 1970; which suggested that if the ascending aorta was involved, surgical treatment (28% mortality) was better than medical treatment (67%), whereas if it was not involved, medical treatment (20% mortality) showed better results than surgical treatment (28%) these recommendations are still being used (Figure 3) [3].
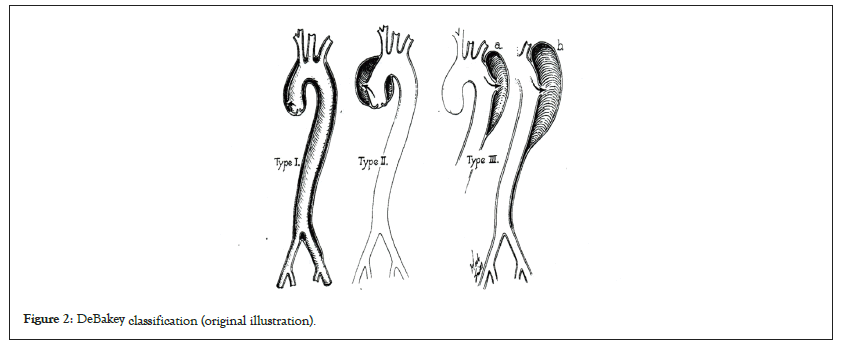
Figure 2: DeBakey classification (original illustration).
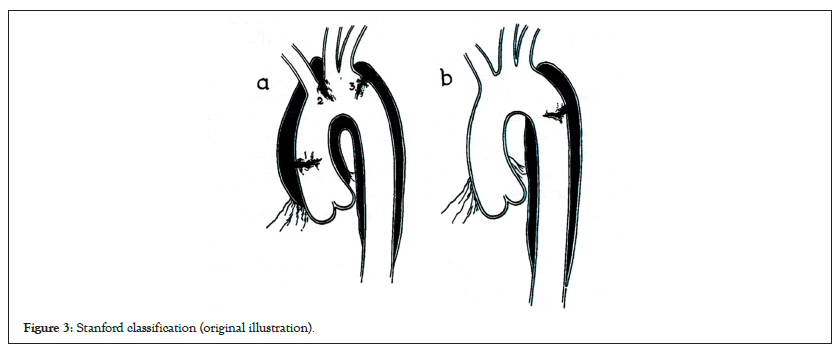
Figure 3: Stanford classification (original illustration).
From the 1970s to the 1990s, open surgical repair was developed as the most reliable method for treating type A AAD. Moreover, cardiopulmonary bypass has improved. A membranous oxygenator was invented to protect blood components. Cardioplegia and other organ preservation techniques have been investigated extensively. Particularly, selective cerebral perfusion was established as a useful method for brain protection [4]. Additionally, well-structured and coated prosthetic grafts, including knitted or woven Dacron®, were successively developed in the 1990s. Simultaneously, component blood transfusions were performed. These developments enabled longer and more complex open surgical procedures.
In 1968, Hounsfield et al. performed X-ray Computed Tomography (CT). Recently, multi detector CT has effectively contributed to the decision-making in AAD treatment. Additionally, echocardiography, especially transoesophageal echocardiography, is useful for intraoperatively investigating the status of AAD and intra cardiac air evacuation.
Optimal medical treatment is essential for AAD management. In the 1960s, the outcomes of medical management that were inferior to surgical outcomes were mainly caused by poor antihypertensive drug selection. Since the 1960s, various antihypertensive drugs, especially β blockers, have been administered for optimal medical management. Currently, various β blockers are available for intravenous and oral use. Furthermore, other types of antihypertensive drugs contribute to improvements in the outcomes of both type A and type B AAD.
During the 20th century, AAD treatment strategies were developed according to the Stanford classification. These were widespread due to their simplicity. In contrast, late outcomes and problems after the initial surgery for type A, or hospitalization at type B onset, were gradually revealed. Nevertheless, the poor prognosis of complicated type B AAD remains unresolved. Open surgical repair of acute complicated type B AAD has shown poor outcomes. Newer strategies are expected to be developed for solving these problems.
Significant change for the first time in 50 Years
In the 2000s, TEVAR, the third treatment option for AAD, was developed. However, the stent structure involved is rigid due to its metallic composition, and TEVAR risks destroying the fragile intima in AAD cases. Additionally, the delivery system for TEVAR carries the potential risk of further disastrous injury due to its thickness and low flexibility.
A stent graft was first developed for true aortic aneurysms. The reinforced radial force and the over-sizing device are important for reducing the gap between the stent graft and native aorta, thereby preventing type 1 endo leaks. Some devices contain hazardous parts, such as large proximal bare stents, barbs, and spines. These structures, which are suitable for true aneurysms may become drawbacks for AAD. Thus, a preliminary stent-graft may not be suitable for AAD.
In the early 2000s, a challenging experience of pilot TEVAR treatment for small cases of complicated type B AAD was reported [5]. This report suggested the possibility of improving the outcome of complicated type B AAD and the need to improve the structure and delivery system of preliminary TEVAR devices. The mortality rate associated with this procedure was 20%, and that of open emergency surgical intervention with descending aorta replacement was ≥ 20%. However, the superiority of TEVAR remains controversial. Currently, advanced TEVAR devices are commercially available, and TEVAR for complicated type B is recommended as Class I in multiple guidelines [6].
False Lumen Expansion (FLE): Residual Problem in Type A and Type B AADs
FLE (>5 mm/6 months) persists even after the initial surgery for type A and after hospitalization at the onset for type B AAD. It may cause aortic rupture. However, this does not always occur in all patients with AAD, and the prediction of FLE is impossible at the onset. Postoperative FLE that required re-intervention occurred in approximately 20% of patients with type A AAD [7]. Furthermore, the long-term outcomes of FLE are worse, and the medical management of type B AAD presents a 30% cumulative mortality rate at 5 years [8]. In other words, majority of the patients with AAD do not show FLE, eliminating the need for further surgical or interventional procedures after initial surgery for type A and hospitalization at onset for type B AAD.
It is important to promptly distinguish between patients with and those without FLE and perform tear closure only in patients with FLE. For example, the strategy to routinely perform total arch replacement using a frozen elephant trunk for all type A AADs (excluding deBakey type II) or routine pre-emptive TEVAR for all type B AAD at onset is over-indicated for patients without FLE. These procedures may increase the possibility of harmful complications.
The INSTEAD-XL trial, a randomized controlled trial conducted in 2013, provided strong evidence of the effectiveness of TEVAR for FLE [8]. It showed that TEVAR for the thoracic descending aorta is sufficient, without the need for additional closure of abdominal distal tears if TEVAR closure is performed 2–52 weeks after onset (clustering at 10–12 weeks). Furthermore, in 2014, the Virtue Registry report showed that the subacute phase (2 weeks to 3 months after the onset) may be optimal for TEVAR [9]. FLE treatment can be performed via TEVAR or open surgical repair. If FLE is detected in the late chronic phase over 3 years from the onset, open surgical repair, such as thoracoabdominal aortic replacement, should be performed because TEVAR may be less effective and can introduce multiple complications in the late chronic phase [9]. Conversely, thoracoabdominal aortic replacement is one of the most invasive procedures with the largest incision among all cardiovascular surgical procedures. Timely and precise TEVAR may be a desirable option for FLE treatment because of its effectiveness and minimal invasiveness.
Although the INSTEAD-XL trial reported that complete thrombosis may invoke re modelling and improve outcomes, I partially agree with these observations. Complete thrombosis of the false lumen may be preferable, but it may lead to occlusion of key branches originating from the false lumen, such as the intercostal artery connecting with the Adamkiewicz artery, and trigger paraplegia. I believe that the objective of TEVAR for FLE is not to cause false lumen thrombosis but to prevent FLE. Therefore, minimal or staged sealing was adopted to avoid paraplegia. Paraplegia is not observed after TEVAR for FLE.
Discussion
Timely and precise TEVAR closing of patent tears causing FLE above the AH
Based on the INSTEAD-XL trial, TEVAR for FLE after the initial surgery was adopted for type A AAD in 2013. Simultaneously, TEVAR for FLE was performed in patients with type B AAD.
There are two important aspects in executing this strategy. First, accurate FLE evaluation using enhanced CT is difficult, but critical in the outpatient unit. The concept of ‘the maximum short diameter’ that is useful for true aneurysm is misleading; FLE may occur in all directions. The FLE should be evaluated from the most detectable view in the FLE direction. The longitudinal diameters of the true and false lumens in the optimal view may be preferable. Further, interlinking of the cardiovascular team members is essential. Initial tear-oriented surgery for type A AAD should be performed in preparation for subsequent TEVAR. The open repair team should understand the meticulous TEVAR planning and procedures for aortic dissections. The TEVAR team should recognize the need for initial aortic surgery. Outpatient doctors should detect FLE in a timely manner. It is important to engage the cardiovascular surgeons familiar with both open aortic repair and TEVAR for this disease.
With the implementation of this strategy, the role of the AH in TEVAR can be noted. In patients with type B AAD with a large false lumen at the AH, a drastic and immediate reduction in retrograde blood flow from the abdominal to thoracic false lumen was observed after TEVAR installation for primary tear closure in the upper portion of the thoracic descending aorta. True lumen recovery after TEVAR may cause prompt shrinkage of the false lumen at the AH and block the retrograde blood flow. Additionally, patients with tears only below the AH after the initial surgery for type A AAD did not show FLE. These observations highlight the importance of timely and precise tear closure above the AH.
In 1965, de Bakey, et al. reported the effectiveness of replacing the descending aorta for de Bakey, type III AAD. They pointed out that the circumferential extent of the dissection process becomes smaller as the descending thoracic aorta approaches the AH. They prevented AH enlargement and achieved good postoperative results using surgical intervention for de Bakey type III AAD. Currently, their recommendations can be followed while performing TEVAR in a less invasive manner.
Recently, the Gore® TAG® Conformable Thoracic Stent Graft with the Active Control System was developed for AAD therapy. It exhibits various advantageous functions and structures. Timely and precise TEVAR closure of tears above the AH that cause FLE will become the gold standard for AAD treatment using this device.
I presented the hypothesis about the role of the AH in type B AAD at the 49th Annual Meeting of the Japanese Society for Cardiovascular Surgery in 2019 as shown in Figure 4, and reported the strategy of combining tear-oriented initial surgery and subsequent TEVAR for type A AAD and the role of the AH in type A AAD in 2023 (Figure 5) [1]. Furthermore, this strategy and its outcome for type B AAD will be presented in the 76th Annual Meeting of the Japanese Association for Thoracic Surgery on 19th to 21st October, 2023.
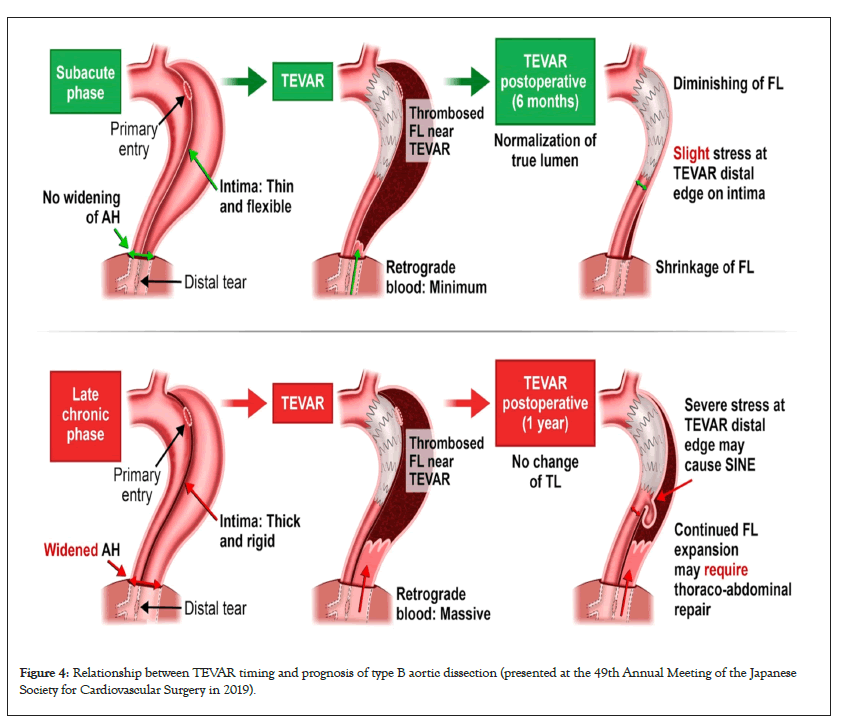
Figure 4: Relationship between TEVAR timing and prognosis of type B aortic dissection (presented at the 49th Annual Meeting of the Japanese Society for Cardiovascular Surgery in 2019).
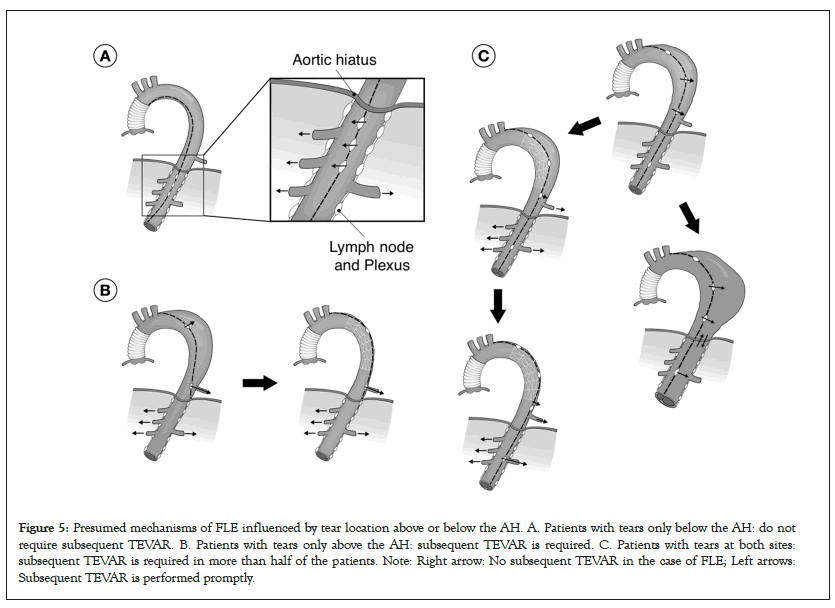
Figure 5: Presumed mechanisms of FLE influenced by tear location above or below the AH. A. Patients with tears only below the AH: do not require subsequent TEVAR. B. Patients with tears only above the AH: subsequent TEVAR is required. C. Patients with tears at both sites: subsequent TEVAR is required in more than half of the patients. Note: Right arrow: No subsequent TEVAR in the case of FLE; Left arrows: Subsequent TEVAR is performed promptly.
Conclusion
The strategy presented herein may resolve issues concerned with FLE during AAD treatment with acceptable outcomes, warranting further investigation. This review will contribute to further developments in this field. True lumen recovery after TEVAR may cause prompt shrinkage of the false lumen at the AH and block the retrograde blood flow. Additionally, patients with tears only below the AH after the initial surgery for type A AAD did not show FLE. These observations highlight the importance of timely and precise tear closure above the AH.
Acknowledgments
I thank Editage (www.editage.com) for English language editing. I appreciate the invitation from the Journal of Vascular Medicine & Surgery to write a mini review for your esteemed journal.
Sources of Funding
This study received no funding.
Conflict of Interest
There are no conflicts of interest to report.
References
- Oda K, Kanda K, Takahashi M, Terao N, Akanuma R, Hasegawa T, et al. Closing of a patent tear above the aortic hiatus and type A aortic dissection outcomes. Ann Thorac Surg Short Reports. 2023;1:375-378.
- de Bakey ME, Walter SH, Cooley DA, Morris GC Jr, Crawford ES, Beall AC Jr. Surgical management of dissecting aneurysms of the aorta. J Thorac and Cardiovasc Surg. 1965;49:130-149.
[Crossref] [Google Scholar] [PubMed]
- Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10:237-247.
[Crossref] [Google Scholar] [PubMed]
- Kazui T, Inoue N, Yamada O, Komatsu S. Selective cerebral perfusion during operation for aneurysms of the aortic arch: A reassessment. Ann Thorac Surg. 1992;53:109-114.
[Crossref] [Google Scholar] [PubMed]
- Duebener LF, Lorenzen P, Richardt G, Misfeld M, Nötzold A, Hartmann F, et al. Emergency endovascular stent-grafting for life-threatening acute type B aortic dissections. Ann Thorac Surg. 2004;78:1261-1267.
[Crossref] [Google Scholar] [PubMed]
- Alfson DB, Ham SW. Type B aortic dissections. Current guidelines for treatment. Cardiol Clin. 2017;35:387-410.
[Crossref] [Google Scholar] [PubMed]
- Halstead JC, Meier M, Etz C, Spielvogel D, Bodian C, Wurm M, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2007;133:127-135.
[Crossref] [Google Scholar] [PubMed]
- Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, et al. Endovascular repair of type B aortic dissection. Long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6:407-416.
[Crossref] [Google Scholar] [PubMed].
- The VIRTUE Registry investigators. Mid-term outcomes and aortic remodeling after thoracic endovascular repair for acute, subacute, and chronic aortic dissection: The VIRTUE Registry. Eur J Vasc and Endovasc Surg. 2014;48:363-371.
[Crossref] [Google Scholar] [PubMed]
Citation: Oda K (2023) Aortic Hiatus and Treatment for Acute Aortic Dissections. J Vasc Surg. 11:539.
Copyright: © 2023 Oda K. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.