Indexed In
- Open J Gate
- JournalTOCs
- The Global Impact Factor (GIF)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 9, Issue 7
An Overview of World-Wide Master Key for Drug Safety Monitoring (Pharmacovigilance) and Its Role in India
Janmejay Pant, Harneet Marwah, Ripudaman Singh* and Subhajit HazraReceived: 19-May-2021 Published: 13-Jul-2021, DOI: 10.35248/2329-6887.21.9.325
Abstract
Pharmacovigilance (PV) described as science & actions connecting to the identification, evaluation, awareness, & mitigation of Adverse Drug Reactions (ADRs) or associated conditions. Some serious cases of ADR contributed to the development of this discipline in the 1970s. There have been many efforts to establish such a programme in India between 1989-2004, but the system has finally begun in 2010 and is functioning successfully and achieving meaningful results. Based on the data gathered via this method, Pharmacovigilance Program of India (PvPI) contributed various data to the World Health Organization (WHO) Uppsala Monitoring Centre (UMC). They also issued some warnings to the stakeholder and made a range of suggestions to the Central Drugs Standard Control Organisation (CDSCO). CDSCO has also advised Marketing Permit Holders (MAHs) to comply with the same requirements and has introduced relevant changes to the Drugs and Cosmetics Act & Regulations. The time has arrived when Indian regulatory authorities would undertake the requisite action on the basis of data produced in our country rather than on the basis of data generated in several other countries
Keywords
ADR; HCP; India; Pharmacovigilance; PvPI; WHO.
Introduction
The word Pharmacovigilance was derived from the two Greek words “Pharmakon”-drug and “vigilare”-to keep observe.
According to WHO, PV is explained as the science & actions connecting to the detection, evaluation, understanding & prevention of ADR’s or associated conditions [1].
In usual, PV is post-market observation. It is important to track medications after they are placed on the market. The factors of consideration can be widely categorised into two groups: [2]
a) Human factor: Among human factors that are of consideration are:
• Inadequate confirmation of safety in the clinical study when it is short-time, mostly lasting just a few weeks.
• Animal tests often do not represent human pharmacodynamics and pharmacokinetics throughout drug development.
• In drug development, the size of the population is restricted and not more than 5000, and sometimes as minimal as 500 participants.
• The demographic group is small and age-specific and genderspecific.
• The studies include narrow guidelines, as only the underlying illness is examined.
b) Post marketing: Several criteria are significant for postmarketing issues. They may be described as follows:
• Post-marketing monitoring provides a diversity of evidence and displaying of the effect of the drug, provided that the drug is used on a broad population scale, with many and complex individual differences playing a role.
• Unwanted adverse effects are frequently observed in Phase IV tests.
• Certain drug-drug interactions, drug-food interactions, intolerance responses, hypersensitivity, severe ADRs such as anaphylaxis that might not be identified in Phase I-III trials are also identified in phase IV trials.
• Patient quality of life control is only feasible in phase IV trials, because it is a long-term analysis. Feedback is also relevant for doctors as well as for the formulating and promoting organization.
• The long-term effectiveness of the medication can be assessed only in the PV research, as all studies up to phase III are shortterm studies in which the long-term efficacy of the drug is impossible to estimate.
The cost-benefit ratio of the treatment can be determined only after phase IV studies. If the drug is to be pulled from the sales due to serious ADRs, the economic strain on the company is immense. In the context of expensive drugs, the influence of medication costs on consumers can only be measured in long-term trials, as with evidence from PV [2].
Necessity of Pharmacovigilance
Medicines are meant to save lives, not steal down. Mortality from a cancer is always possible, but mortality from a drug is not appropriate. In the United States of America (USA), ADRs are some of the 10 largest reason of death [3] and in the United Kingdom, it is proposed that ADRs may trigger 5,700 fatalities per year. The number of hospital referrals attributed to drug-related incidents in certain nations is about 10%. [2]
A variety of ADR linked to medications have led to the establishment of the “Pharmacovigilance” science. The thalidomide tragedy of 1961 is one of the events where thousands of congenitally deformed children were born. This inspired the WHO to carry out a rigorous review of ADR medicines, which is the origin of PV. Several of ADRs have been identified thereafter, and some of those are presented in below (Table 1) [2].
| S.No | Drugs | Examples of severe and unpredictable adverse effects | Year |
|---|---|---|---|
| leading to the cessation of prescription | |||
| 1 | Veralipride | Anxiety, Depression and Movement disorders | 2007 |
| 2 | Rofecoxib | Cardiovascular effects | 2004 |
| 3 | Terfenadine | Torsade de pointes | 1997 |
| 4 | Benoxaprofen | Nephrotoxicity and Cholestatic jaundice | 1982 |
| 5 | Practolol | Sclerosing peritonitis | 1975 |
| 6 | Clioquinol | Subacute nephropathy | 1970 |
| 7 | Thalidomide | Phocomelia | 1961 |
Table 1: Some severe and unpredictable adverse events leading to withdrawal of medicine
Why ADR monitoring in India?
In addition to cultural, dietary, disease prevalence and genetic distinctions, changes in prescription patterns influence the benefit-risk ratio, e.g. Pioglitazone is prohibited in certain emerging regions due to increased incidence of bladder carcinoma, while it is not prohibited in India due to low incidence of bladder carcinoma [2].
History of Pharmacovigilance in India
In the Before Independence Age of Pharmacovigilance: Until 1947
In year 1880, the opinion of the Glasgow board formed by the British Medical Association considered that ‘Chloroform was hazardous for cardiovascular and in contrast, much harmful co ether’. Then after counter argument was given after 8-year by Surgeon – Major Laurie, head of state Hyderabad Medical School in year 1888, who demonstrated the effectiveness of chloroform in approximate 40 000 patients with no single mortality in Hyderabad City [4]. They never seen the cardiovascular disorder or critically damaged by it. This was possibly the 1st implementation of a systemic technique for the identification of ADRs in the world [5].
Post Freedom, Pharmacovigilance: 1947 to 1970s
The 1st PubMed nation indexed case report on ADRs emerged in 1975, when 336 individuals underwent to the general medical unit were prospective study screened for ADRs and 20 percent of them were observed to acquire ADRs [6].
Pharmacovigilance Programme Progression in India: 1983 To 2009
ADR surveillance service head to the Dr. Molly Thomas, a pioneering clinical pharmacologist at Christian Medical College, Vellore, South India, who developed the programme in year 1983 [7]. They trained 1,650 doctors and set up a spontaneous tracking plan in the institute. In one year, they got 600–900 data and issued 4 yearly newsletters. They was the 1st to recognise weak monitoring frequency and clinicians’ propensity to record only significant and not small responses [5].
Unlike developing countries, many of them adopted structured PV programmes in year 1960s after the Thalidomide tragedy, India’s PV Policy launched in year 1986. That was the 1st effort by the regulatory agency in India to officially create PV in nation. System foreseen the development of knowledge on ADR by monitoring randomly & had an aggregate of 12 major centres (several they based in Chandigarh, Delhi, Kolkata, Lucknow, Mumbai and Pondicherry) distributed throughout the India. Primary health facilities and hospitals had to be connected to such major hubs. It was projected that each major centre will have a demographic of 50 000 000 [8].
In year 1997, the programme underwent a major amendment, with India being section of the WHO International Drug Surveillance System organised by the UMC in Sweden. That was the 2nd effort by the agency to reinvigorate PV in the nation. That model of the service had 6 centres around country Aligarh, Chandigarh, Kolkata, Lucknow, Mumbai, New Delhi and Pondicherry [9]. None of the 2 try in a year 1980s and year 1990s truly gave some substantial boost to the country’s discipline development, with most of the documenting being both limited and intermittent.
Those 2 efforts, the Indian Medical Research Council, India’s leading research organization, conducted its own Adverse Reaction Control System, which was monitored by the working group. The independent system has a separate set of 12 clinics around the India which reported 4490 adverse effects in 82,761 patients shown in the centres [10,11]. When the initiative was ongoing, the working group identified crucial areas that required change if the initiative were to be implemented. This included the required choice of centres involved in project, the requirement for specialized submission and maintenance of data personnel, consistent recognition of the coordination centre and instruction in analysis and interpretation of data [5].
A massive shift towards keeping India a major key in PV around the world came in year 2004 to year 2005, with aid by the worldbank & WHO seeking to lay its support beneath India. The Indian PV Programme was formally christened India’s National PV Program (NPP) on 23 Nov 2004 & World Bank committed USD 100 000 a year for 5-year [12]. This system supervised through the National PV Advisory Committee, central agency body in India. The India’s adverse reaction cases flowed to 2 major zones – the South West Zonal Centre and the North East Zonal. The South West Zonal Centre will be gathering its self-data and will have 3 regional centres monitoring to it. Likewise, the North East Zonal Centre will gather its self-data & have 2 territorial centres to report to it. In exchange, every regional centre will have branch centres reporting data [13].
The initiative has 3 broad objectives: to promote an environment of monitoring in the brief, to disseminate data & to engage wide number of health providers in meantime, and to set universal targets as a long-duration goal. Although that revived initiative was with its share of obstacles. Funding by the World Bank alone without agency help implied that programme stayed in ‘project mode,’ there were no governance at the peak, there was no clear interaction between the centres collaborating with the agency, & there was no effect on legislative decision-making. Investment by the World Bank ended in year 2009 [14].
Pharmacovigilance Programme in India: 2010 to Till
On 14 July 2010, the regulatory made a deliberate attempt to formalise PV in the nation [15]. There was a secure structure with the agency at the forefront, official regulations in effect to sustain initiative, a national centre based at the Pharmacology department, the All India Institute of Medical Sciences (AIIMS), New Delhi, and government resources to build the long-term programme. The aims of this programme were multiplier in order to produce knowledge on ADR in the community of Indians; to increase understanding between medical services practitioners of the importance for PV; to actively track the profit risks of medicinal products; to establish unbiased, impartial, evidence-based guidelines on the protection of medicinal products; to urge regulators to pursue safety-related judgements; Convey results to all relevant investor and set up a National Centre of Excellence in accordance with national wide guidelines for drug safety surveillance. Four stages of the initiative were already Emergence foreseen (year 2010 to year 2011); growth & restructuring (year 2011 to year 2012); expansion and maintenance (year 2012 to year 2013); expansion and optimisation (2013–2014); and perfection (2014–2015). The programme has an advisory group & a task force providing strategic guidance to the regulatory and 3 specialist committees (quality analysis, signal evaluation & key training panels) for recommendations on difficulties:
• The Committee of Quality Control regularly evaluate performance & accuracy of the ICSRs, suggest decisions to the PvPI the participating committees further an information processing, & develop frameworks & policy records for followup activities.
• The Committee of Signal to understanding & analyse the signals provided by ICSRs for interpretation and actionable measures and recommends relevant regulatory actions to CDSCO.
• The Core Training Council evaluates coaches, requirements practicing & practicing material and communicates with global organisations on the involvement & execution of PV practicing programmes.
Legislation In India Pharmacovigilance
Schedule Y
The legal criteria in India for PV are regulated through the provisions of Schedule Y of the Drugs and Cosmetics Act 1945. Schedule Y includes laws covering preclinical and clinical trials for production of developing medicines, specifications for clinical trials for the producing and of new drugs in India. Schedule Y was modified & updated on 20 Jan 2005 as the proceed pledge of the Drug Controller General of India to verify that the PV responsibilities of pharmaceutical firms are sufficiently complied with. [16] An effort was created in revised Schedule Y to further describe the functions & obligations of pharma firms in relation to their drugs and also the monitoring of adverse effects from clinical trials [17].
Unexpected Responses of Drug
Schedule Y states that any cases with significant unintended adverse reactions should be notified to the licencing agency within 15 days of the individual’s primary reception of data, with follow-up data given. Individual ADR data should be required in the next annual safety summary report, and not generally as a matter of urgency. However more information on the identification, analysis and ADR follow-up was not discussed in Schedule Y. Accordingly, medical firms in India follow International conference on Harmonisation (ICH) E2D guidelines for the management of spontaneous data on goods [17].
Pharmacovigilance System Workflow as per Indian Scenario
Pharmacovigilance Programme of India
The regulatory agencies soon recognised the need for a comprehensive PV programme to protect community welfare, and the NPP was change its title India’s Pharmacovigilance Program (PvPI), that began running on 14 July 2010, with the AIIMS, New Delhi, as the National Coordinating Centre (NCC). In track ADRs across nation, the system had 22 ADR Monitoring Centres (AMCs), along with AIIMS. Subsequently, the NCC was moved from AIIMS to the Indian Pharmacopoeia Commission (IPC), Ghaziabad, on 15 April 2011, for the successful execution of the initiative, with the key objective of producing empirical data on drug safety in line with global drug safety surveillance requirements. Since PV was perceived to be a service that tracks medications for harmful drug events and dosage mistakes, some physicians became worried about it and they thought that their skills were being challenged [18]. The PvPI is working hard to address this obstacle of uncertainty and to minimize the causes for undercounting by implementing a variety of clinical practice, awareness-raising and practicing activities for Health Care Professionals (HCPs) on a continuous justification to teach and remind them of the practice of reporting ADRs. HCPs have been made clear that no disciplinary action is taken with the monitoring of ADRs [19].
The purpose of the programme was to create faith comparison the practitioner and the patient, thus improving care & enhancing people’s interest in the nation’s health system. The PvPI partners with the project to promote the safety of the Indian people by maintaining that the pros of drugs outweigh the risk related with their use [20].
The PvPI gathers data obtained in the shape of ICSRs from AMCs, HCPs, and other non-HCPs. PvPI assesses the evidence and uses the findings to suggest educated regulatory interventions.
Around the same time, it reminds HCPs and customers of the risks associated with pharmaceutical drugs. In addition, the PvPI also seeks to detect under-standard drugs and prescribe, dispense and distribute errors in order to increase consumer health. Around the same period, the PvPI aims to solve other issues such as illicit medicines, antimicrobial tolerance and monitoring during vaccines and other regional programmes [21].
PVPI’s Aims and Strategies
The main PVPI goals are as follows:
• Build a strategic plan for patient care monitoring
• Recognise and interpret new emerging data from documented cases
• Evaluating the benefit-risk balance of products on the market
• Produce Scientific proof research on efficacy of pharmaceutical products
• To assist governing bodies in Strategic thinking mechanism on the utilise of medicines
• Convey safety data on the utilise of pharmaceutical products to different stakeholders in order to reduce risk
• To develop as a regional area of competence for PV practises
• Cooperation with other regional knowledge sharing and data processing centres;
Offer educational and advisory service to individuals’ regional PV centres around the globe [20, 21].
Framework and Structure of the PVPI
At the period of its establishment, the PVPI was planned to be done in 3 stages. 1st Phase enabled development of 40 AMCs and launched in 2010. Eventually, 140 Medical Council of India approved medical academics were connected to initiative such branch of 2nd Phase in year 2011. Eventually, 3rd Phase will include whole healthcare system by year 2013. The 1st phase also consisted with 2 stages, 1sta phase & 1stb phase. These all were meant to improve AMCs with regard to new facilities. Logistic and technical assistance from the CDSCO zoning centres based in Ghaziabad, Mumbai, Kolkata and Chennai are provided to the AMC [22,23]. The CDSCO Zonal Centres are under the execution of the CDSCO Headquarters (See Figure 1).
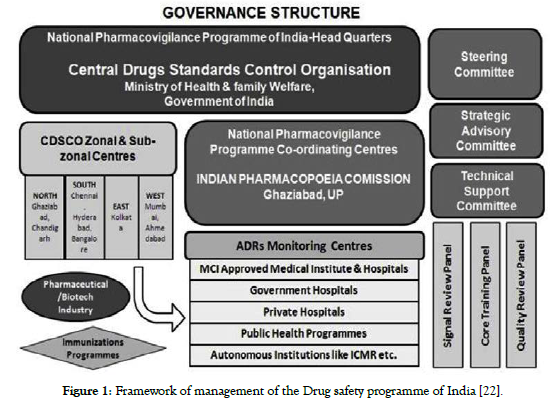
Figure 1. Framework of management of the Drug safety programme of India [22].
Adverse Drug Reaction Data Flow
The ADR information sent to ADR Monitoring Centre (AMC’s) to be subject to primary review by the PV personnel. AMC personnel will hold a list of each operation executed. Upon receipt of the ADR forms, a causal assessment will be carried out by the coordinating centre before the records are submitted to the PV database. The consolidated ADR report will be compiled by the coordinating centre at pre-planned time periods. The combined data will then be transferred via the VigiFlow database to the UMC ADR portal & the signal analysing to be performed [22,24]. The ADR communication channel can be schematically depicted according to below presented in (Figure 2 and Figure 3).
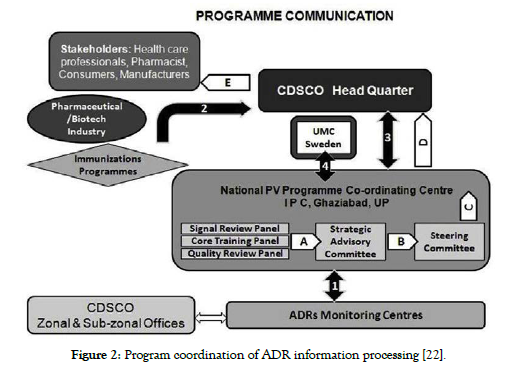
Figure 2. Program coordination of ADR information processing [22].
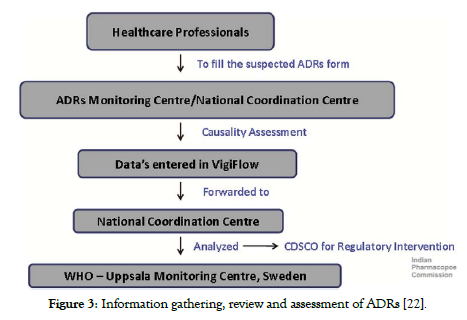
Figure 3. Information gathering, review and assessment of ADRs [22].
Unpredictable ADR Monitoring and ADR monitoring form could be reporting in 2 ways.
1. Active Surveillance Monitoring
2. Passive Surveillance Monitoring [22]
Achievements of the PvPI
• Development of 250 AMCs which provide platform for PV & monitoring environment across India [21].
• A variety of AMC awareness campaigns have been initiated for public outreach. The ADR monitoring environment required to develop the framework is growing around the world, even within categories such as nursing workers and MAH. [25] Monitoring forms have been accessible in 10 vernacular languages and different template for HCPs & customers are also accessible on the CDSCO website.
• PvPI applied to regional wellbeing services like measles, neglected tropical infections, vector-borne illness, Acquired immunodeficiency syndrome and immunisation. Product warnings produced for medications have been transmitted to their respective platforms for enhanced public health results. Post-compatibility with the post-immunization ADR gained greatly in the National Regulatory Authority’s Assessment of 2017, when the surveillance benchmarking method hit the maximum maturity stage of IV.
• PvPI has introduced a ‘Basic & Regulatory Dimensions of PV’ skill learning initiative to educate young PV practitioners. HCPs from across India joined the program.
• Resistance of antimicrobial in nation is growing and the PvPI works by any indicates necessary to monitor the possibility of resistant bacteria. PV measures are also being enhanced at state, local and country level to maintain patient care. The 2017 regional healthcare agenda, initiated by the Ministry of Health and Family Welfare (MoHFW), examines infectious diseases and PV. This regulation amendment would have scope for effective monitoring and avoidance of ADRs whenever possible.
• The PvPI under the auspices of the IPC, MoHFW, was thoroughly investigated by the WHO – National Regulatory Authority (NRA) 2017 Global Benchmarking Tool (GBT). As a result, diligence reached a maturity growth of 4 out of 5 [26,27].
• In consideration of performance and nature of employ carried out by the NCC-PvPI period the last 6‑year & it is important support to WHO Programme for International Drug Monitoring, the IPC currently known as the WHO Coordinating Centre for PV in Public Health agencies and Regulatory Systems [28].
Contrast Between Us, Eu And Indian Pv
PV is also in its development stage in India relative to the PV program in each the EU and the US. Both EU and USA need mandatory oversight of all significant ADR, while in India there has been no clear requirements for monitoring of ADRs apart from Schedule Y before PvPI welcomed into force.28 It has officially become mandatory for MAHs to register ADRs into PvPI in 2018. MedWatch and EudraVigilance are the US and EU web-based ADR monitoring function, Although India works on Vigiflow based on the WHO framework. ADR forms are not categorised in 2 distinct categories, as in the US and EU schemes [29-30].
Asvice About Reportions
What to Report?
Report serious ADR: There is a serious reaction once the patient’s result is
• Mortality
• Frightful for life
• Primary and long-term hospitalisation
• Minor, recurrent or long lasting disability
• Congenital anomaly
• Needed action to avoid permanent injury
• Document non-serious, not known repeated or unusual ADRs related to pharmaceutical drugs, vaccines and herbal products.
Who can Report?
• All health care practitioners can report ADRs.
Where to Report?
• The thoroughly completed Suspicion ADR notification form could be submitted to the neighbouring AMC or individually to National Co-ordinating Centre (NCC).
• To notify ADRs, dial Helpline (Toll Free) 1800 180 3024.
• Or mail completed form directly to pvpi@ipcindia.net or pvpi. ipcindia@gmail.com
• There is number of AMCs in India is accessible at: http:// www.ipc.gov.in, http://www.ipc.gov.in/pvpi/pv_home.html.
What happens to the Submitted Iinformation?
• The details contained in the template is treated in absolute assurance. The usefulness determination is conducted on AMCs utilizing the WHO-UMC metric. The evaluated form is sent to the NCC via the ADR database.
• Eventually, the information are processed & sent to the Global PV Database maintained by the WHO.
• The documents are routinely checked by NCC-PvPI. The knowledge produced depends on these observations, it improves the formative evaluation of the benefit-risk ratio of medicinal products.
• The data shall be forwarded to the PVPI Advisory Board established by the MoHFW. The Commission shall be responsible for analysing results and recommending any measures that could be needed [31].
Mandatory Field for Suspected ADR Monitoring Form
Patient initial, age at start of response, term of reaction, data of start of reaction details, Suspicion of medication(s) and reporter details [32].
Management of the Safety Database
No detailed guidelines on the monitoring of safety records or the preservation and upgrading of Company Core Data Sheet or Company Core Safety Information is available from Indian regulators. Usually, all evidence gathered during literature review, spontaneous adverse case events, clinical and non-clinical studies or from all other sources are recorded and stored in the drug safety file [32,33].
Discussion
PV proceeds to play a vital role in overcoming the threats raised by the ever-enhancing variety and efficacy of drugs, many of which have an inherent and often uncertain possibility for badness. When side effects & risk occur, particularly during newly identified, it is important that they be recorded, examined and accurately conveyed to a public with awareness of the understanding of the facts. There is an exchange between the benefits and the possibility for risk for all pharmaceutical goods. Damage will be reduced by confirming that drugs of high consistency, protection and effectiveness are used rationally and that the customer's needs and interests are taken into consideration when making clinical judgments. To do this it is to promote global welfare and to promote a sense of faith among patients in the medications they use that will raise faith in the healthcare service in usual, confirm that risks in the usage of drugs are expected and handled, provide authorities with the necessary evidence to update the guidelines on the use of medicines, and increase collaboration among health practitioners and educating the public and health practitioners to consider the usefulness or harm of the medications they recommend.
Conflict of Interest
No potential conflicts were declared.
Author's Funding
No financial support of the research, authorship, and/or publication of this article was declared.
Authors Contribution
• Janmejay Pant and Harneet Marwah conceived of the presented idea and both developed the theory and performed the computations.
• Ripudhaman M Singh encouraged Janmejay Pant and Harneet Marwah to investigate this work and supervised the overall this work.
• Subhajit Hazra reviewed the final internal draft and gave valuable suggestions.
All authors discussed the conclusion and contributed to the final manuscript.
REFERENCES
- The Safety of Medicines in Public Health Programmes: Pharmacovigilance an essential tool. World Health Organ. 2006;7(2):1-34.
- Mandal SC, Mandal M. Evolution of pharmacovigilance programme: Present status in India. Pharma Times 2017;49(5):31-36.
- Lazarou J, Pomeranz BH, Corey PN, Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998;15;279(15):1200-1205.
- Thomas KB. Chloroform: commissions and omissions. Proc. R. Soc. Med. 1974;67:723–730
- Thatte UM, Chaudhary N, Gogtay NJ. Pharmacovigilance program of India: history, evolution and current status. Adverse Drug React Bull 2018;213:1-4.
- Vakil B, Kulkarni R, Chabria N. Intense surveillance of adverse drug reactions: an analysis of 338 patients. J Clin Pharmacol 1975;15(5-6):435–441.
- World Health Organisation. The hidden costs of essential medicines in Essential Drugs Monitor. Essent Drugs Monit. 2003;33:12
- Kulkarni RD. Reporting systems for rare side effects of nonnarcotic analgesics in India. Problems and opportunities. Med Toxicol 1986;1 (Suppl 1):110–113.
- Protocol for National Pharmacovigilance Program. CDSCO, ministry of health and family welfare, government of India; 2004
- Rahman SZ. Book review on ADR – adverse drug reactions.
- Dikshit RK, Desai C, Desai MK. Pleasures and pains of running a pharmacovigilance center. Indian J Pharmacol 2008;40:S31–S34.
- Adithan C. National pharmacovigilance programme. Indian J Pharmacol. 2005;37(6):347
- Biswas P, Biswas AK. Setting standards for proactive pharmacovigilance in India: the way forward. Indian J Pharmacol 2007;39(3):124–128.
- Pharmacovigilance Programme of India. The journey travelled and the way forward. WHO Drug Information. 2018;32:10–17.
- Kalaiselvan V, Jai Prakash, Singh GN. Pharmacovigilance program of India. Arch. Pharm. Pract. 2012;3(3):229–232.
- CDSCO (n.d.b) Central Drugs Standard Control Organisation. Schedule Y (Amended version) 2005 [Internet]. Accessed on 26 April 2021.
- Biswas P. Pharmacovigilance In India. Mann’s Pharmacovigilance2014:285-289.
- Dhamija P, Kara S, Sharma PK. Indian College of Physicians (ICP) position statement on pharmacovigilance. J Assoc Physicians India. 2017;65:63.
- Rakesh KR, Rakesh KP, Anil B. Under reporting of ADRs by medical practitioners in India—results of pilot study. Adv Pharmacoepidem Drug Saf 2012;1(3):1-3.
- Pharmacovigilance Programme of India. Introduction & functions. 2017.
- Kalaiselvan V, Srivastava S, Singh A, Gupta SK. Pharmacovigilance in India: Present Scenario and Future Challenges. Drug Saf 2019;42(3):339-346.
- Dylan Fernandes S, Anoop NV, Castelino LJ, Narayana Charyulu R.A National approach to pharmacovigilance: The case of India as a growing hub of global clinical trials. Res Social Adm Pharm 2019;15(1):109-113.
- Rama P, Rodrigues PA, Georgy A. Pharmacovigilance: perspectives and future challenges in Indian scenario. Asian J Pharm Clin Res 2011;4(4):1-4.
- Suke SG, Kosta P, Negi H. Role of pharmacovigilance in India: an overview. Online J Public Health Inform 2015;7(2):1-34.
- PvPI. Important events. PvPI Newslett 2016;6(15):4-18.
- Singh GN. Director’s message. PvPI Newslett 2017;7(18):4-20.
- WHO. NRA meets WHO standards on vaccine regulation. PvPI Newslett 2017;7(18):4-20.
- Postigo R, Brosch S, Slattery J, van Haren A, Dogné JM, Kurz X et al. EudraVigilance medicines safety database: publicly accessible data for research and public health protection. Drug saf. 2018;41(7):665-675.
- Jose J, Rafeek NR. Pharmacovigilance in India in comparison with the USA and European Union: challenges and perspectives. Ther Innov Regul Sci 2019;53(6):781-786.
- Bhargavi H, Jadav Krupa C, ThulaDilip G, Maheswari R. Regulatory requirements of Pharmacovigilance system and its comparison in India and USA. J Glob Trends Pharm Sci 2015;6(1): 2351-2356.
- Miller V, Nwokike J, Stergachis A. Pharmacovigilance and global HIV/AIDS. Curr Opin HIV AIDS 2012;7(4):299-304.
- Kalaisevan V, Kaur I, Singh S, Singh GN. Pharmacovigilance programme of India: System put in place to report adverse drug reactions. Indian J. Pharm. Educ. Res2016;50(1):S212-214.
- Arora D. pharmacovigilance obligations of the pharmaceutical companies in India. Indian J Pharmacol 2008;40(Suppl 1):S13-S16.
Citation: Pant J, Marwah H, Singh RM, Hazra S. (2021). An Overview of World-Wide Master Key for Drug Safety Monitoring (Pharmacovigilance) and Its Role in India, J. Pharamacovigil. . 9:325. doi-10.35248/2329-6887.21.9.325.
Copyright: ©2021 Singh R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

