Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- SWB online catalog
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2025) Volume 14, Issue 3
Adoption of Pharma 4.0 from Industry 4.0 and Automation of Quality Assurance
Mritunjay Kumar1, Preeti Patel2 and Balak Das Kurmi3*2Department of Pharmaceutical Chemistry, ISF College of Pharmacy, GT Road, Moga-142001, Punjab, India
3Department of Pharmaceutics, ISF College of Pharmacy, GT Road, Moga-142001, Punjab, India
Received: 23-Nov-2023, Manuscript No. PDS-23-24037; Editor assigned: 27-Nov-2023, Pre QC No. PDS-23-24037 (PQ); Reviewed: 12-Dec-2023, QC No. PDS-23-24037; Revised: 03-Feb-2025, Manuscript No. PDS-23-24037 (R); Published: 13-Feb-2025
Abstract
Over the last two and half centuries the pharmaceutical industries has grown from handmade herbal medicines to sophisticated and fully automated manufacturing units. Pharma Industry 4.0, also known as smart manufacturing, incorporates new technologies into the pharmaceutical production process, such as the Industrial Internet of Things (IIoT), real time QMS control systems, Cyber Physical Systems (CPS) and incorporation of robots with Artificial Intelligence (AI), and Industry 4.0 concepts. The paper aims to share the latest development of advanced technology that can be implemented in present day pharmaceutical manufacturing industries in compliance with regulatory authorities. By evaluating case best practices and current trends, this research will provide insights into the benefits, obstacles, and consequences of adopting Industry 4.0 technologies into quality assurance processes. The outcomes of this study will help us understand how Pharma 4.0 may affect quality assurance in the pharmaceutical industry, resulting in higher product quality, regulatory compliance, and patient safety.
Keywords
Pharma 4.0; Pharma 3.0; Automated quality assurance; Automated documentation; Regulatory challenges; Challenges
Introduction
Over the years, the pharmaceutical sector has seen significant improvements and transformations driven by technical innovations and shifting regulatory needs. With the introduction of Industry 4.0, a new wave of automation and digitalization has changed production processes in a wide range of industries. In this context, the pharmaceutical industry is adopting Pharma 4.0, a customized adaption of Industry 4.0 concepts, to improve operational efficiency, product quality, and regulatory compliance [1]. It optimizes and automates important operations by leveraging cutting edge technologies like as the Internet of Things (IoT), big data analytics, Artificial Intelligence (AI), robots and machine learning. Pharma 4.0 has the potential of not only improving production processes but also transforming quality assurance techniques in the pharmaceutical business by seamlessly integrating these technologies. The pharmaceutical sector has traditionally depended heavily on manual inspection and testing, which may be time consuming and prone to human mistake. The use of automation and modern analytical tools, on the other hand, provides a chance to improve the accuracy, efficiency and consistency of quality assurance operations. It will provide a number of advantages, including real time monitoring and management of production activities, early detection of quality issues, and increased speed and precision of quality evaluations. These developments can assist in ensuring products meet demanding quality standards as well as compliance requirements [2]. However, implementing Pharma 4.0 and automating quality assurance comes with challenges. For successful deployment, technical challenges, data integrity and security, regulatory compliance, and worker skill gaps must be overcome. Furthermore, the complexity of pharmaceutical production processes, as well as the requirement for comprehensive validation methods, needs a cautious approach to implementing these disruptive technologies. The purpose of this study is to investigate the implementation of Pharma 4.0 concepts and the automation of quality assurance in the pharmaceutical business [3]. This research will give insights into the advantages, challenges, and consequences of incorporating Industry 4.0 technology into quality assurance methods by reviewing case best practices, and current trends. This study's findings will help us understand how Pharma 4.0 may change quality assurance in the pharmaceutical sector, resulting in better product quality, regulatory compliance, and patient safety.
Literature Review
Gaps of pharma 3.0 and pharma 4.0
Pharma 3.0 gaps: Pharma 3.0, which represents traditional pharmaceutical production, has a number of flaws that limit its efficiency and competitiveness. Using manual techniques results in inefficiencies and longer drug development delays. Inconsistent product quality can also be caused by variances introduced by human interaction. Limited automation adds to greater operating expenses and an inability to keep up with the industry's fast improvements [3,4]. List of related gaps are as follows:
• No interconnected factory facility.
• Partial automation of industrial equipment’s.
• Submission of written documents and forms to regulatory authority.
• Problems and hazards in field operation.
• Single unit stabilization at a time.
• Operation management.
• Handwritten manual SOP.
• Blending technique and proportion control.
Pharma 4.0 gaps: Pharma 4.0, defined by modern technology, is not without its obstacles. The initial expenditure necessary to deploy innovative technology might be prohibitively expensive, especially for smaller pharmaceutical businesses. Because of networked systems and digital data interchange, there is an increased danger of data breaches and cyber-attacks. Regulatory alignment is difficult since authorities may struggle to keep up with the rapid innovations of pharma 4.0. Up-skilling the staff and addressing any resistance to change are two more critical areas that must be addressed for effective adoption [3–5]. List of related gaps are as follows
• High investment and operation cost.
• Leadership and change control.
• Coordination between departments.
• Skilled personals required.
• Concern about cyber security.
• Regulatory challenges.
• Ethical considerations.
• IT risks.
Assessment and mitigation of challenges
In Pharma 3.0: Pharma 3.0 refers to the period of the pharmaceutical business preceding the current revolution of Pharma 4.0, which integrates new technology and data driven methodologies. During this time, the industry faced challenges such as longer drug development schedules, higher prices, restricted data access, partial automation, manual documentation, hazards in field operation, etc. [3]
No interconnected factory facility: An interconnected factory will provide reliable and high speed means of communication between different machines and system in entire manufacturing premises. Ethernet is used to connect factory machines and system together for agility and can be easily expanded and upgraded to accommodate new machines or system in the factory. It’s cost effective compared to other networking technology. Ethernet network can be secured using encryption, access control and security measures. This can help in preventing unauthorized access and protects sensitive data from cyber threats. It provides flexibility to employees and supervisor to access data wherever they are [6].
Partial automation of industrial equipment’s: Fully automated industrial equipment will reduce the need of human interventions, resulting in faster and more accurate quality production process. It can perform tasks with greater precision and consistency than humans, resulting in higher product quality and lower rejection rate. It will lower labor expenses by removing the need for human operators as well as material waste and energy consumption. Automated equipment can execute jobs that people might find risky or hazardous, lowering the risk of workplace accidents and injuries. This is especially critical in the pharmaceutical business where certain chemicals and procedures may be extremely harmful [7].
Submission of written documents and forms to regulatory authority: Written documents although widely used, can be time consuming to compile and prone to human error. Realtime data submission involves the immediate transfer of data as it is generated, allowing for a dynamic and continuous monitoring of operations. Real time data provides regulators with up to the minute insights into the manufacturing processes, enabling proactive identification and resolution of issues. It also offers the opportunity for real time quality control and risk assessment. While written documents provide a detailed retrospective analysis, real time data submission offers the advantage of agility, responsiveness, and the ability to capture critical deviations promptly [8].
Problems and hazards in field operation: Field operations present various challenges and hazards to human workers, ranging from physically demanding tasks to exposure to hazardous environments. These risks can include heavy lifting, repetitive motions, exposure to extreme temperatures, chemicals, or radiation, repairing narrow area machines and potential accidents. Integrating robotics into field operations can provide effective solutions to mitigate these problems. Robots can handle physically strenuous tasks, reducing the risk of injuries to human workers. They can operate in hazardous environments, eliminating the need for human exposure. Robots equipped with sensors and advanced algorithms can perform precise and delicate tasks, minimizing errors and improving overall safety. Additionally, human workers can collaborate with robots, working alongside them to enhance productivity and efficiency. The integration of robotics in field operations not only addresses the hazards faced by human workers but also enhances productivity, accuracy, and overall safety [9].
Single unit stabilization at a time: Traditionally individual units such as machines or equipment were operated and monitored independently, leading to inefficiencies and difficulties in stabilization. With Pharma 4.0 automation, these units can be integrated into a connected ecosystem where they communicate and share real time data. Through the use of sensors, data analytics, and artificial intelligence, the system can monitor and control each unit, ensuring optimal operation and stabilization. By analyzing data from multiple units simultaneously, the automation system can identify patterns, predict deviations, and automatically adjust operations to maintain stability. This integrated approach eliminates the stratified operation of individual units, leading to improved efficiency, reduced downtime and enhanced overall stability in the pharmaceutical manufacturing process [10].
Operation management: In Pharma 3.0 there are stratification among the organization, time consuming and sub-optimal decision making. There’s high dependency on individuals capabilities and potential risk of human error during operation management. By the means of automated operation management system, operations are managed in a highly efficient and interconnected manner. Real time data acquisition, analysis, and predictive modeling enable proactive decision making, ensuring optimal utilization of resources, improved production planning and enhanced product quality. Automation plays a pivotal role as robotics, AI and IoT devices work in sync to streamline workflows, minimize human error and increase productivity. Additionally, advanced analytics help identify bottlenecks, optimize supply chain management, and enable predictive maintenance. This comprehensive approach to operation management in the next generation pharma industry maximizes efficiency, reduces costs, and fosters innovation, enabling pharmaceutical companies to meet the evolving demands of the market effectively.
Handwritten manual Standard Operating Procedures (SOP): Its time consuming for supervisor of operation to handwrite and maintain SOP. It doesn’t updates and doesn’t include all operator know how. Advantages of manual SOPs are limited and it can’t prevent major human errors. The use of electronic Standard Operating Procedures (e-SOPs) offers several advantages over traditional manual SOPs. e-SOPs provide a digital and centralized platform for creating, managing, and distributing SOPs throughout an organization. They offer greater accessibility, allowing authorized personnel to access and review SOPs from anywhere, anytime. e-SOPs also facilitate easier updates and version control, ensuring that employees have access to the most up-to-date procedures. The digital format allows for interactive elements such as hyperlinks, videos, and images, enhancing comprehension and clarity. Additionally, e-SOPs can track and record user interactions, providing an audit trail for compliance purposes. Overall, e-SOPs streamline the SOP lifecycle, improve accessibility and usability, and contribute to more efficient and compliant operations within an organization [11].
Blending technique and proportion control: Manual blending technique involves the manual mixing of ingredients or components in a manufacturing process. It typically requires human operators to measure and manually add the desired proportions of each ingredient. While this method may be suitable for small scale operations, it is prone to variations in proportions, human error, and time consuming processes. In contrast, automatic blending technique with proportion control leverages technology to automate the blending process. It utilizes specialized equipment and control systems to precisely measure and mix ingredients in the desired proportions. Sensors and feedback mechanisms ensure accurate proportion control, minimizing errors and variations. This automated approach significantly improves consistency, efficiency, and accuracy in blending operations. It also enables real time monitoring and adjustment, ensuring optimal product quality and reducing the reliance on manual intervention.
In pharma 4.0
Pharma 4.0 reflects the pharmaceutical industry's cutting edge revolution, employing sophisticated technology. While the benefits of Pharma 4.0 are significant, its implementation comes with challenges that must be analyzed and addressed. The pharmaceutical sector can unleash the full potential of Pharma 4.0 and drive itself towards a more sustainable and patient centric future by identifying and overcoming challenges relating to data security, workforce up skilling, regulatory compliance, and the integration of innovative technology [5].
Human capital risk: Human capital risk is the lack of relevant skills and competencies among employees, which can hinder the organization's ability to adapt to technological advancements and changing industry demands. One approach is to invest in workforce training and development programs to ensure employees are equipped with the necessary skills to operate and maintain automated systems effectively. By providing comprehensive training, employees can adapt to new technologies, understand the details of automated processes and troubleshoot issues that may arise. Additionally, fostering a culture of continuous learning and innovation encourages employees to stay updated with industry advancements. Another strategy is to establish robust safety protocols and ergonomic measures to protect workers from potential hazards associated with automated machinery. This includes implementing safety sensors, barriers and regular equipment maintenance. Furthermore, promoting a healthy work life balance and creating a supportive work environment can contribute to employee satisfaction and reduce burnout. By addressing human capital risks through training, safety measures and a positive work culture, the automated pharmaceutical manufacturing industry can enhance employee well-being, productivity and overall operational success.
Change management risk: Change management risk refers to the potential negative outcomes or adverse effects that can arise during the process of implementing organizational change. These risks can include resistance from employees, inadequate communication and disruption of operations, increased costs, decreased productivity and even failure to achieve desired outcomes. One key approach is to conduct thorough planning and analysis before implementing any changes. This involves identifying potential risks, assessing their impact and developing mitigation strategies. Engaging stakeholders, including employees, in the change process through effective communication and training helps to create awareness and alleviate resistance. Additionally, implementing change in a phased manner allows for gradual adaptation and reduces the chances of major disruptions. Regular monitoring and evaluation of the change process help to identify any unforeseen risks or issues, allowing for timely adjustments. Finally, having a contingency plan in place ensures that any unexpected challenges can be addressed promptly, minimizing the impact on production and overall operations. By employing these change management risk mitigation strategies, the automated pharmaceutical manufacturing industry can navigate transitions effectively and maximize the benefits of automation while minimizing potential risks.
Cyber security: Cyber security risk mitigation is crucial in the automated pharmaceutical manufacturing industry to protect sensitive data, critical systems, and intellectual property from cyber threats. One key aspect of cyber security risk mitigation is implementing robust security measures at various levels. This includes deploying firewalls, encryption, access controls and intrusion detection systems to safeguard networks and systems from unauthorized access. Regular security audits and vulnerability assessments help identify and address potential weaknesses. Employee training and awareness programs play a vital role in promoting good cyber security practices and minimizing human related risks. Additionally, implementing secure software development practices and regularly updating and patching software and systems help prevent exploitation of vulnerabilities. Establishing incident response plans and backups aids in timely detection and recovery from cyber-attacks. Collaborating with industry experts and staying abreast of emerging threats and best practices is essential for maintaining a strong cyber security posture. By incorporating these risk mitigation measures, the automated pharmaceutical manufacturing industry can minimize cyber threats and protect sensitive information and critical operations.
Regulatory challenges: The adoption of Pharma 4.0, which involves the integration of sophisticated technology into pharmaceutical production, offers various regulatory challenges that must be solved in order for the initiative to be effective. The incorporation of modern technology into pharmaceutical production raises various regulatory hurdles when implementing Pharma 4.0. Among these challenges are ensuring data integrity and security, adhering to changing regulations, validating and qualifying automated systems, achieving interoperability between different technologies, providing adequate workforce training, adapting auditing and inspection processes, and ensuring supply chain transparency. To address these difficulties, pharmaceutical businesses, regulatory agencies, and technology suppliers must work together to develop comprehensive compliance policies and standards that ensure patient safety, product quality and data integrity in the changing Pharma 4.0 landscape.
IT risks: The deployment of Pharma 4.0, which combines sophisticated technology into pharmaceutical manufacturing, introduces a number of IT risks that must be addressed in order for the projects to be successful and secure. Data breaches and cyber-attacks are among the primary IT risks in Pharma 4.0, since the growing use of networked systems and digital technologies increases the susceptibility of sensitive information. To protect against illegal access and data manipulation, businesses must prioritize data integrity and security, establishing robust data management systems and encryption measures. Interoperability issues between various software and devices can be dangerous, requiring careful consideration and compliance with regulatory guidelines. Furthermore, establishing worker expertise in running and maintaining automated systems becomes critical to reducing the risk of errors and potential IT related interruptions. To effectively manage these IT risks in the context of Pharma 4.0, proactive risk mitigation methods, close engagement with IT security professionals and regular monitoring and assessment of IT infrastructure are required.
Limit of coding: The complexity and intricacy of laws and standards controlling pharmaceutical goods is the primary coding barrier in the automated pharma production business. To provide accurate and traceable data across the supply chain, the use of automated coding systems for product identification, labeling and serialization necessitates careful adherence to industry rules and regulatory regulations. Any mistakes or discrepancies in the coding process might have major ramifications, including as product recalls, supply chain interruptions, and even harm to patients. As a result, the pharmaceutical sector must invest in strong coding technology and implement tight validation and quality control methods to reduce the risk of coding mistakes and preserve regulatory compliance. Furthermore, due to the ever changing nature of pharmaceutical legislation and worldwide serialization obligations, a flexible coding infrastructure capable of responding to future changes and enabling smooth interface with other automated systems in the manufacturing process is required.
Protocol for automated quality assurance
It outlines the particular methods and needs for exploiting sophisticated technologies in quality control. The protocol addresses issues such as data gathering, processing, and interpretation, as well as smart sensor integration and real time monitoring. It also tackles regulatory compliance, data integrity and validation methods to assure automated QA systems' dependability and traceability. Following the protocol allows pharmaceutical organizations to implement standardized and efficient methods that optimize QA processes, improve product quality, and embrace the benefits of automation in the context of Pharma 4.0 [12].
Define quality requirements: Establish definite quality criteria and requirements for the pharmaceutical product or process that is being automated. Criteria including purity, potency, stability, safety and efficacy could be part of this.
Develop an automated quality assurance system: Create and put into action an automated quality assurance system that can track, manage and check crucial quality criteria while they are being manufactured or tested. Utilizing cutting edge sensors, software or analytical tools for data collecting, analysis and decision making may be required.
Validation and calibration: Verify the accuracy, dependability and consistency of the automated quality assurance system's measurement and analysis of the quality parameters. This might entail doing validation studies, performance certification, and continuing instrument or sensor calibration.
Standard Operation Procedure (SOP): Creating and documenting standard operating configuration, operation, maintenance, trouble shooting and data management should all be covered in SOPs.
Data management and analysis: Establish a strong data management system to collect, store and analyze the data produced by the automated quality assurance system. To find patterns, trends and possible problems, this may involve data validation, data integrity checks and data trend analysis.
Real time monitoring and control: Implement real time monitoring and control mechanisms as part of the automated quality assurance system to continually monitor important quality metrics throughout the production or testing process. In order to take prompt corrective action when quality parameters are out of specification, this may entail putting up alarms or warnings.
Documentation and record keeping: System configuration, calibration, validation, SOPs and data records are all tasks associated to the automated quality assurance system that require thorough documentation and record keeping. This is crucial for audit trails, traceability and regulatory compliance.
Training and competency: Provide the staff members engaged in running, maintaining and troubleshooting the automated quality assurance system with proper training and competence assessments. This could entail instruction on data analysis, system functioning and troubleshooting techniques.
Continuous improvement: Through recurring reviews, audits, and process improvement projects, continuously monitor, evaluate and enhance the effectiveness of the automated quality assurance system. This may entail locating and resolving the underlying reasons for quality deviations, putting corrective and preventative measures in place and promoting a continuous improvement culture.
Regulatory compliance: Ensure that the automated quality assurance system conforms to all essential regulatory standards, including Good Manufacturing Practices (GMP, Good Laboratory Practices (GLP and other related guidelines and regulations.
Automation of quality assurance
It encompasses the use of robotics, artificial intelligence, machine learning, smart sensors, and real time monitoring to improve efficiency, accuracy and compliance. Automated quality assurance systems manage vast amounts of data, execute complicated analysis and allow for speedier decision making while decreasing human error and unpredictability. Pharmaceutical firms may boost productivity, product quality and operational agility by embracing automation. Steps to automation of quality assurance are.
Use machine learning to identify defects: Free numbers in learning model using images of defective and nondefective product. The model can then be used to analyze image of products in real time and detect any defects.
Utilize robotics: Use robots to perform repetitive tasks such as inspecting products for defects or measuring dimensions. This can improve the accuracy of measurements and reduce the risk of human error.
Implement continuous integration and deployment: Use Continuous Integration and Deployment (CI/DI) tools to automate the process of testing and deploying software. This can help to identify defects early in the development cycle and reduce the time it takes to deploy new features.
Implement automated testing: Use automatic testing software to test applications and software products. This can help to identify bugs and defects early in development cycle and reduce the need of manual testing.
Use IoT device to monitor quality: Use IoT device to monitor the quality of product in real time. This can help to identify any defects or issues early in the production process and reduce waste.
Implement Statically Process Control (SPC): Use SPC to monitor the quality of products in real time. SPC can help to identified trends and patterns in production data and alert operators when there are divisions from expected quality standards [13].
Protocol for automated documentation
A protocol for automated documentation establishes a systematic framework for creating, managing, and preserving documentation via the use of automated procedures and technology. The protocol specifies particular principles and processes for creating automated systems for data collecting, organization and storage. This includes the use of techniques like natural language processing, machine learning and templates to automate the development of documents like reports, records and guides. To assure the quality and dependability of automated documentation, the protocol also handles data integrity, version control and security precautions. Steps to protocol for automated documentation
Define document control requirements: Establish outlined document control standards and requirements, including document types, formats and naming conventions, version control and document retention guidelines for pharmaceutical documentation.
Employ a document management system: Opt for a reliable and legal document management system with features like version control, document routing, electronic signatures, audit trails and security controls that is tailored to the demands of the pharmaceutical sector.
Document creation and evaluation: Using the specified document management system, create and evaluate pharmaceutical documents such as Standard Operating Procedures (SOPs), batch records, protocols, reports and other relevant documents. In accordance with the defined document control standards, make sure that all documents are prepared, reviewed and approved by authorized people.
Release and approval of documents: Before releasing documents for usage, ensure that they have received the necessary approvals. According to the specifications for document control, this may entail electronic signatures or other approval procedures. Release the papers for usage in the document management system once they have been authorized.
Document distribution and access: Establish a regulated procedure for document distribution and access to guarantee that the appropriate employees have access to the correct version of the documents at the appropriate time. This may entail granting employees access permissions to documents, alerting staff to their availability and making sure that outdated or superseded documents are deleted or archived.
Document revision and change control: Control document modifications and alterations in compliance with the defined guidelines for document control. To do this, it may be necessary to initiate and record document modifications, send files for review and approval, update document metadata and keep a transparent audit trail of all document changes.
Training and competency: Ensure that the personnel responsible for the development, review, approval and dissemination of documents have the necessary training and skills to carry out their assigned duties. This could entail instruction on document control protocols, how document management systems work and how to create and evaluate documents.
Document retention and archiving: Establish document retention and archiving procedure that conforms with all applicable internal regulations and regulatory requirements. This may include setting up document retention policies, controlling and securely storing records and assuring that preserved documents can be retrieved when required.
Regulatory compliance: Ensure that the automated document management system conforms to all applicable rules and guidelines, including Good Documentation Practices (GDP), Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP) and others.
Periodic audits and reviews: Conduct routine audits and evaluations of the automated document management system to check for compliance with defined document control standards, spot any deviations or problems, and promote continuous development.
Automation of documentation
Document automation is using technology and software to automate and accelerate the process of producing, maintaining, and organizing various sorts of documents. This involves automating the development of reports, records, contracts, and other written documents utilizing technologies like document generating software, templates, data integration and artificial intelligence. Large volumes of data may be captured and processed effectively by automated documentation systems, eliminating manual work and potential human mistakes. Furthermore, to assure the correctness, integrity, and confidentiality of the papers, these systems frequently contain version control and data security features [14]. Steps to automation of documentation are
Employ document management software: To assist automate the process of production, storing and exchanging documents among divisions inside a firm. This makes it simple to maintain document versions, track changes and collaborate with others. Some document management software that can be used for audit management, training management, records and electronic signature in market are MasterControl, Veeve Vault, OpenText Documentum, SAP Document Management System, EtQ Reliance, etc.
Incorporate electronic signatures: By removing the requirement for physical signatures on documents, electronic signatures can save time and prevent mistakes. This is especially valuable in areas that demand a lot of paperwork, such as healthcare and law.
Use templates: You may automate the process of producing new documents by developing templates for popular types of paperwork including contracts, purchase orders and invoices. By doing this, time is saved and accuracy and consistency are guaranteed in the papers.
Use Optical Character Recognition (OCR): make advantage of OCR technology to automatically extract data from documents like receipts and invoices. When compared to manually entering the data, this can save time and minimize mistakes.
Use Chatbots: it may be used to automate the process of addressing typical documentation inquiries, such as how to fill out a form or where to find a certain document.
Use Artificial Intelligence (AI): AI can be utilized to automatically identify and categories papers, extract data from documents and even construct documents depending on certain criteria.
Audit checklist of automated documentation system
An audit checklist for an automated documentation system is a thorough list of criteria and standards used to analyze the automated documentation system's effectiveness, compliance and dependability. The checklist often includes a variety of system elements, such as data integrity, security, version control, user access, validation and regulatory compliance. It guarantees that the automated documentation system fulfills industry and regulatory standards while also offering accurate, consistent and dependable document management capabilities. The audit checklist assists auditors and organizations in identifying potential flaws or areas for improvement in the automated documentation system, ensuring that it functions efficiently and meets the organization's overall objectives (Table 1) [15].
| Area of audit | Audit questions | Compliance outcome (Y/N) |
|---|---|---|
| Document control | Are the electronic documentation system validated according to regulatory requirements? | |
| Is there an established document control process in place? | ||
| Are documents reviewed and approved before release? | ||
| Are obsolete document removed from circulation? | ||
| Is there a master list of all controlled documents easily identifiable and accessible? | ||
| Electronic signature | Are electronic signatures used for approving documents? | |
| Are electronic signature records maintained? | ||
| Are the procedures for the use of electronic signatures clearly defined? | ||
| Are the controls for electronic signatures sufficient to ensure their authenticity, integrity and confidentiality? | ||
| Is there a process for revoking electronic signatures in case of misuse? | ||
| Version control | Are document versions tracked and maintained? | |
| Is there a process for version control and version history? | ||
| Are the latest versions of documents easily accessible? | ||
| Is there a system in place for notifying employees of changes to documents? | ||
| Are previous versions of documents archived and easily retrievable? | ||
| Data integrity | Are electronics records and signatures maintained with appropriate controls? | |
| Is data integrity ensured during data entry, processing and review? | ||
| Are there procedures in place for detecting and investigating data discrepancies? | ||
| Is there a system for identifying and preventing unauthorized modification to data? | ||
| Is there a system for data backup and recovery? | ||
| Audit trial | Are audit trials established for electronic records and signatures? | |
| Are audit trials accessible and complete? | ||
| Is there a system for reviewing audit trials reports? | ||
| Are there procedures in place for detecting and investigating discrepancies in audit trails? | ||
| Is there a process for periodically reviewing audit trail reports? | ||
| Retention and destruction | Are electronic records retained and destroyed according to company policies and regulatory requirements? | |
| Is there a system in place for maintaining and archiving electronic records? | ||
| Is there a process for identifying and retaining critical records? | ||
| Is there a process for securely destroying electronic records? | ||
| Is there a process for reviewing and revising retention and destruction policies? | ||
| Security | Is access to electronic records and signatures controlled? | |
| Are security measures in place to prevent unauthorized access or modification? | ||
| Are access controls reviewed periodically? | ||
| Is there a process for identifying and addressing security incidents? | ||
| Are security procedures documented and communicated to employees? | ||
| Training | Are employees trained on the use of electronic documentation systems? | |
| Are employees trained on the use of electronic signatures? | ||
| Are employees trained on the document control process? | ||
| Are employees trained on data integrity and audit trail procedures? | ||
| Is there a process for periodic refresher training on electronic documentation systems? | ||
| System validation | Are the electronic documentation systems validated according to regulatory requirements? | |
| Is there documentation available to demonstrate the system validation process? | ||
| Is there a process for periodic revalidation of the electronic documentation system? | ||
| Are changes made to the electronic documentation system validated before implementation? | ||
| Backup and recovery | Is there a process for backing up electronic documentation data? | |
| Is the backup process documented and tested? | ||
| Is there a process for recovering electronic documentation data in the event of a system failure? | ||
| Is the recovery process documented and tested? | ||
| Remote access | Is remote access to the electronic documentation system allowed? | |
| Are there controls in place to ensure secure remote access? | ||
| Is remote access to the electronic documentation system audited and monitored? | ||
| Is remote access to the electronic documentation system restricted to authorized personnel only? | ||
| Third-party documentation | Are third-party documents used in the electronic documentation system controlled and tracked? | |
| Is there a process for verifying the accuracy and integrity of third-party documents? | ||
| Are third-party documents approved before they are used in the electronic documentation system? | ||
| Are there controls in place to ensure that third-party documents do not compromise the security or integrity of the electronic documentation system? | ||
| Change control | Is there a process for managing changes to the electronic documentation system? | |
| Is there documentation available to demonstrate the change control process? | ||
| Are changes made to the electronic documentation system approved and documented before implementation? | ||
| Is there a process for reviewing and assessing the impact of changes to the electronic documentation system? | ||
| Disaster recovery | Is there a disaster recovery plan in place for the electronic documentation system? | |
| Is the disaster recovery plan documented and tested? | ||
| Are there measures in place to ensure business continuity in the event of a disaster? | ||
| Are there procedures for restoring electronic documentation system data in the event of a disaster? |
Table 1: Checklist for automated documentation system audit.
Options for real time data storage and retrieval
The automated pharmaceutical manufacturing sector demands reliable and efficient real time data storage and retrieval options to ensure streamlined operations, compliance with regulations and overall quality control. There are various methods for data storage and retrieval, each one having its own set of benefits and drawbacks [16]. Among the most common choices are:
In-Memory Databases (IMDB): IMDBs are essential in the automated pharmaceutical production companies because they provide fast data access and real time processing capabilities. IMDBs allow exceptionally quick access and retrieval by storing data directly in main memory (RAM, which is crucial for time sensitive decision making in pharmaceutical production. This enables effective process monitoring, control and optimization, improving overall product quality and compliance with regulatory standards. SAP HANA, Redis and Memc ached are some examples of popular IMDBs. While IMDBs provide considerable speed advantages, they may necessitate a major investment in memory resources as well as comprehensive data backup mechanisms to assure data permanence and security [17].
Time Series Database (TSDB): TSDBs efficiently manage time stamped data collected during the manufacturing process. These databases are built to manage massive amounts of data points that are indexed by time, allowing for real time data storage and retrieval. Monitoring and interpreting time-sensitive data is critical in the pharmaceutical sector for guaranteeing product quality, safety and regulatory compliance. InfluxDB, OpenTSDB and TimescaleDB are some examples of TSDBs that provide high performance storage and retrieval, making them ideal for processing data from diverse sensors, equipment and systems in an automated manufacturing environment. They make real-time monitoring, process optimization and predictive maintenance easier by providing instant access to previous and current data. Manufacturers may use TSDBs to discover patterns, detect abnormalities, and make data driven choices to enhance efficiency and save costs. In conclusion, Time Series Databases are valuable tools for the automated pharmaceutical manufacturing sector, allowing for the effective administration of time sensitive data and enabling real-time analytics for improved decision making and process optimization.
Cloud-based storage: Cloud based storage has become an increasingly popular choice in the automated pharmaceutical manufacturing industry due to its scalability, flexibility and ease of integration. Manufacturers may benefit from real time data access, automated backups and a variety of analytics tools that can assist monitor and improve the production process by employing cloud platforms such as Amazon Web Services (AWS), Google Cloud Platform (GCP), or Microsoft Azure. This provides increased productivity, adherence to industry laws and better decision making based on real time data. Furthermore, cloud based storage provides cost effective solutions that can be rapidly changed to suit the expanding demands of a pharmaceutical facility, without requiring major upfront expenditures in on premises hardware and infrastructure [18].
Relational Database Management System (RDBMS): It provides a dependable and well-structured solution to store and handle complex data. RDBMS uses tables such as MySQL, PostgreSQL, and Microsoft SQL Server to arrange data into rows and columns, maintaining data consistency and integrity via the use of primary and foreign keys, constraints and triggers. RDBMS are extensively used and trusted for processing sensitive data, despite being slower than in-memory databases. RDBMS may be used in the pharmaceutical sector to store data about raw ingredients, production processes, equipment maintenance, product batches and quality control, among other things. RDBMS also provide for quick querying and reporting, which is critical for monitoring, analysis and compliance with industrial requirements. RDBMS may not provide the fastest real time data access, but they remain a popular choice for enterprises looking for a tried and true data management solution [9].
Edge computing: It brings data storage and processing closer to the point of data collection, lowering latency and boosting real time decision making. Data may be stored and processed locally by using edge devices such as IoT gateways, reducing the requirement for continual connectivity with central servers. This strategy improves manufacturing processes' responsiveness and efficiency, allowing for speedier quality control and greater adherence to safety regulations. Furthermore, edge computing relieves network congestion and lowers data transmission costs, making it an important solution for improving data management in the pharmaceutical business [20].
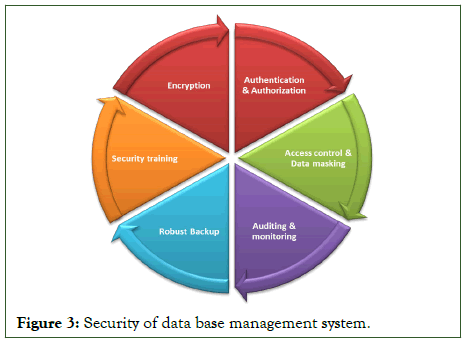
Security of data base management system
Security is critical in the automated pharmaceutical manufacturing industries, where sensitive data and intellectual property must be protected from unauthorized access and possible threats. The security of a Database Management System (DBMS) is vital in preserving critical information and maintaining regulatory compliance.
Authentication and authorization procedures are critical for restricting DBMS access. Multi-Factor Authentication (MFA), for example, ensures that only authorized users may access the system. Role based access control enables granular permissions, providing users access only to the data and functionality required for their respective jobs. This prevents unauthorized users from gaining access to critical information.
Encryption is critical for data security, both at rest and in transit. By encrypting data, it becomes unintelligible to unauthorized parties even if it is accessed or intercepted. This safeguarding measure protects confidential information, such as drug formulations, manufacturing processes and patient data, from unauthorized viewing or tampering.
In the pharmaceutical sector, regulatory compliance is crucial. DBMS security should be in accordance with laws such as FDA 21 CFR Part 11 and EU GMP Annex 11. This includes features like secure electronic signatures, audit trails and access restrictions for tracking and monitoring data access and changes. Organizations may demonstrate their commitment to data integrity and compliance by adhering to these laws.
Access control and data masking techniques are employed to limit access to sensitive data within the DBMS. Organizations guarantee that only authorized individuals may access and edit vital data by adopting stringent access controls. Data masking obscures sensitive information by substituting fictional or jumbled values. This safeguards data during non-production usage or while exchanging information with third parties, lowering the danger of data breaches.
Auditing and monitoring are one the important for detecting potential security breaches and policy violations. Monitoring database activities such as user actions and system performance on a regular basis enables firms to quickly discover any odd activity or unwanted access attempts. Audit logs give a trail of evidence, which aids investigations and ensures responsibility.
A robust backup and disaster recovery strategy is essential for mitigating risks and ensuring data protection. Regular backups, securely kept off-site, maintain data availability and allow for recovery in the case of data loss or system failure. Disaster recovery plans include the processes required to restore data and resume activities with as minimal disruption as possible.
Organizations may safeguard the network infrastructure surrounding the DBMS by deploying extensive network security measures such as firewalls and intrusion detection systems. This gives an extra layer of protection against external attacks.
Security training and awareness programs can be held to educate employees about data security best practices including proper handling of sensitive information, password management and phishing prevention. Organizations can limit the risk of human mistakes compromising DBMS security by establishing a security conscious culture.
Regular security assessments, such as vulnerability scanning and penetration testing, aid in identifying and correcting any security vulnerabilities in the DBMS and infrastructure. These preventative approaches enable firms to patch vulnerabilities before attackers may exploit them.
An integrated approach to DBMS security is critical for automated pharmaceutical industry. Organizations can ensure the integrity, confidentiality and availability of their data, protect critical information and meet regulatory requirements by combining strong authentication, encryption, access controls, auditing, backup and recovery, network security, employee training and regular assessments.
Results and Discussion
SWAT analysis
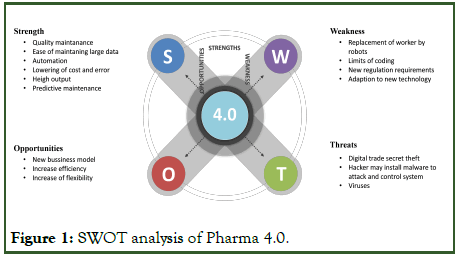
SWOT analysis is a strategic technique for assessing the strengths, weaknesses, opportunities, and threats of a particular program or sector, such as Pharma 4.0, which combines new technology into pharmaceutical production. Figure 1. Illustrates about SWAT analysis of Pharma 4.0.
Strengths: Pharma 4.0 leverages modern technologies such as big data analytics, artificial intelligence, and the Internet of Things to provide major benefits to the pharmaceutical sector. Automated procedures provide increased productivity, faster data analysis and better decision-making, resulting in more efficient manufacturing and a shorter time to market for new pharmaceuticals. The combination of real time monitoring and predictive maintenance results in increased operational efficiency and cost effectiveness. Furthermore, Pharma 4.0 encourages an innovation and digital transformation culture, propelling the sector toward a more flexible and adaptive future.
Weaknesses: Despite its potential, Pharma 4.0 has limitations. Some pharmaceutical firms, particularly smaller ones, may be put off by the initial expenditure necessary for technology adoption and infrastructure changes. Furthermore, the difficulty of integrating numerous systems might cause implementation issues and significant interruptions throughout the shift. The sector may also face opposition from its current workforce, demanding substantial training initiatives to prepare personnel for the digital era.
Opportunities: The use of modern technology allows for data driven decision making, helping businesses to optimize production processes, increase product quality and improve patient safety. Real time data gathering and analysis facilitate customized medical techniques, resulting in more effective therapies and better patient outcomes. Pharma 4.0's linked nature fosters cooperation and data exchange among stakeholders, opening the path for more effective supply chain management and smooth regulatory compliance.
Threats: Pharma 4.0 is not without threats. Because of growing connections, data security and privacy issues have arisen, demanding sophisticated cybersecurity measures to protect sensitive patient information and intellectual property. Because of the rapid speed of technological innovation, certain technologies may become outdated or require regular upgrades, necessitating ongoing investments. Furthermore, regulatory frameworks must be aligned with fast emerging technology, since regulators must keep up with advancements while assuring patient safety and product quality (Figure 1).
Figure 1: SWOT analysis of Pharma 4.0.
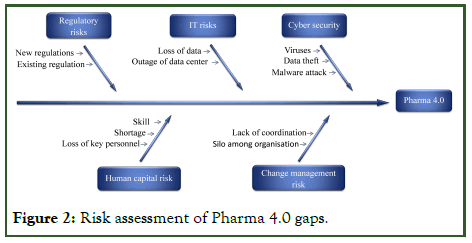
Risk assessment and mitigation of pharma 4.0
Pharma 4.0 risk assessment entails identifying, assessing, and evaluating possible hazards connected with the deployment of modern technology in pharmaceutical manufacture. The risk assessment results show both the potential and challenges that Pharma 4.0 provides to the business.
The risk assessment reveals a number of hazards linked with Pharma 4.0. Data security and privacy issues are among the primary threats, since the linked nature of Pharma 4.0 raises the potential of data breaches and cyberattacks. Because of the quick speed of technological innovation, there is a danger of investing in technologies that may become obsolete in the future, perhaps resulting in financial losses. Integrating several technologies can add complications and implementation issues, possibly interrupting existing workflows and operations. Furthermore, for certain pharmaceutical organizations, the initial expenditure necessary for technology adoption and employee training may pose a major financial risk.
The study goes deeper into each highlighted risk, examining its possible impact on the pharmaceutical business and recommending mitigation solutions. To handle data security and privacy issues, adopt strong cyber security safeguards, encryption methods, and access restrictions to secure sensitive information. Staying current with technological developments and using scalable and adaptable solutions can help reduce the risk of investing in outmoded technologies. Collaboration with technology vendors and good planning might assist ease implementation issues during Pharma 4.0 deployment. Investing in comprehensive staff training programs may reduce resistance to change and ensure employees are well equipped to adopt new technology. Figure 2 illustrates risk assessment of Pharma 4.0 (Figure 2).
Figure 2: Risk assessment of Pharma 4.0 gaps.
Automated quality assurance and documentation
The automated quality assurance approach has resulted in improved product quality, consistency, and compliance throughout the pharmaceutical production processes. Companies have decreased the risk of human mistakes and unpredictability by employing automated technologies, resulting in enhanced overall product quality and patient safety. Real time data collection and analysis have aided in data-driven decisionmaking, allowing for the proactive discovery and resolution of quality concerns throughout the production process.
Furthermore, the protocol has accelerated product release by automating validation and quality control operations, lowering time to market and improving operational efficiency. Simultaneously, the automated documentation procedure has transformed how pharmaceutical businesses handle documentation operations. The introduction of the protocol has greatly reduced human labor and mistakes in document production and administration. Intelligent automation has been facilitated through the use of modern technologies such as natural language processing and machine learning, allowing for quicker processing of massive amounts of data. Additionally, the protocol has better data integrity and version control, guaranteeing that correct and up-to-date information is readily available. Furthermore, computerized documentation has improved regulatory compliance by simplifying audits and inspections and lowering the risk of noncompliance.
This study focuses on the strategic benefits and problems observed throughout the automated QA and documentation protocol implementation. It underlines the significance of selecting appropriate technologies that correspond with the pharmaceutical production environment's particular demands and processes. Collaboration with technology suppliers and internal stakeholders is key to ensure the automated systems' effective integration and acceptability. It also recognizes the necessity for ongoing monitoring and assessment to ensure the protocol's success. Regular audits and evaluations are required to uncover possible flaws and opportunities for improvement in automated procedures. Ongoing staff training and up skilling are critical to ensuring that personnel are well-equipped to use automated technologies efficiently and effectively handle any resistance to change.
Real time data storage, retrieval and security
Because of the use of real time data storage and retrieval capabilities in DBMS, companies may now capture, process, and retrieve data in real time. This has resulted in speedier decision making and more adaptability to shifting business situations. Real time data retrieval guarantees that consumers have access to the most recent information, allowing for more accurate and informed decision making. Furthermore, real time data storage has increased data consistency and accuracy, lowering the danger of data inconsistencies and mistakes. The introduction of strong security measures in the database management system has been critical in protecting sensitive data from unauthorized access and cyber threats. To safeguard data at rest and during transmission, encryption mechanisms, access restrictions, and user authentication procedures have been implemented. The security features of the DBMS have greatly decreased the danger of data breaches and unauthorized alterations, assuring data privacy and regulatory compliance.
The study focuses on the strategic value of real time data storage and retrieval in relational databases. It stresses the advantages of using this technology in a variety of industries, including banking, healthcare, and e-commerce, where rapid access to reliable information is crucial for operational efficiency and consumer pleasure. Businesses have gained a competitive advantage by being able to adapt quickly to market movements and client needs. It also emphasizes the necessity of a multilayered strategy to DBMS security. Regular security audits, vulnerability assessments and penetration testing are critical for identifying and mitigating any flaws. Employee education on cyber security best practices and data handling protocols is critical for mitigating internal security concerns. In addition, the debate emphasizes the need of disaster recovery and backup procedures in ensuring data availability in the case of system failures or cyber events. Figure 3 illustrates Security options for data base management system.
Figure 3: Security of data base management system.
Conclusion
Automation simplifies and speeds quality assurance by allowing for real time monitoring and decision making. It improves accuracy and consistency, lowering the risk of human mistake and assuring regulatory compliance. Furthermore, automation improves documentation processes, resulting in more efficient record keeping and traceability, both of which are critical in assuring product quality and safety. However, resolving hurdles such as data security and staff up-skilling is required for successful deployment. However, the potential gains in productivity, cost effectiveness, and product quality make Pharma 4.0 and automation of quality assurance and documentation mandatory for pharmaceutical manufacturers seeking to remain competitive and deliver innovative, safe and reliable products to meet future demands. Real time data storage, retrieval and analysis enable more efficient and accurate documentation procedures, resulting in greater transparency and traceability of pharmaceutical operations. Automated documentation eliminates the possibility of human mistake and allows for the prompt capture of vital information, assuring regulatory compliance. However, the rising dependence on digital documentation raises concerns about data security and integrity. To secure sensitive information and ensure regulatory compliance, pharmaceutical businesses must prioritize sophisticated cybersecurity protections and data protection policies. Overall, the incorporation of Pharma 4.0 documentation technology allows the industry to improve regulatory standards, streamline compliance processes, and ultimately offer safer and more effective pharmaceutical goods to patients.
Acknowledgments
The authors are highly thankful to ISF College of Pharmacy, Moga, Punjab for providing platform and facilities for research work. Contribution of Computer lab for providing computer system and facilities is highly acknowledged.
References
- Rüßmann M, Lorenz M, Gerbert P, Waldner M, Justus J, Engel P, et al. Industry 4.0: The future of productivity and growth in manufacturing industries. BCG.2015;9(1):54-89.
- Schmidt A, Frey J, Hillen D, Horbelt J, Schandar M, Schneider D, et al. A framework for automated quality assurance and documentation for pharma 4.0. InComputer Safety, Reliability, and Security: 40th International Conference, SAFECOMP 2021, York, UK, 2021, Proceedings 40 2021 (pp. 226-239). Springer International Publishing.
- Arden NS, Fisher AC, Tyner K, Lawrence XY, Lee SL, Kopcha M. Industry 4.0 for pharmaceutical manufacturing: Preparing for the smart factories of the future. Int J Pharm. 2021;602:120554.
[Google Scholar] [PubMed]
- Chen W, Park SJ, Cheng TN, Lau NW, Khaw LF, Leeâ?ÂÃÂ?ÂÂLane D, et al. Pharmaceutical industry: Challenges and opportunities for establishing pharma 4.0. Industry 4.0 Vision for Energy and Materials: Enabling Technologies and Case Studies. 2022:313-337.
- Ozcan KN, Yesilyurt O, Demir S, Konuk B. Industry 4.0 concepts, technologies, and its ecosystem. InIndustry 4.0: Technologies, Applications, and Challenges 2022 (pp. 1-33). Singapore: Springer Nature Singapore.
- Lu Y, Morris KC, Frechette S. Current standards landscape for smart manufacturing systems. National Institute of Standards and Technology, NISTIR. 2016; 8107(3).
- Haleem A, Javaid M, Singh RP, Rab S, Suman R. Hyperautomation for the enhancement of automation in industries. Sens Int. 2021; 2:100124.
- Chen Z, Xia H. Crossdata: Leveraging text-data connections for authoring data documents. CHI '22: Proceedings of the 2022 CHI Conference on Human Factors in Computing Systems, New Orleans, USA. 2022:1-15.
- Rasmussen J. Risk management in a dynamic society: a modelling problem. Saf Sci. 1997;27(2-3):183-213.
- Kundur P. Power system stability. Power system stability and control. 2007;10:7-1. [Crossref]
- Sandle T. Good documentation practices. J Valid Technol. 2014.
- Schreibmann E, Elder E, Fox T. Automated quality assurance for imageâ?ÂÃÂ?ÂÂguided radiation therapy. J Appl Clin Med Phys. 2009;10(1):71-79.
[Crossref] [Google Scholar] [PubMed]
- Winkler D, Biffl S. Improving quality assurance in automation systems development projects. Quality Assurance and Management. 2012;23:20-40.
- Caldas CH, Soibelman L. Automating hierarchical document classification for construction management information systems. BAS. 2003;12(4):395-406.
- Linna A, Korhonen M, Airaksinen M, Juppo AM. Experiences of using the GMP audit preparation tool in pharmaceutical contract manufacturer audits. Drug Dev Ind Pharm.2010; 36(6):632-637.
[Crossref] [Google Scholar] [PubMed]
- Bock C. Scheduling and Execution of Genome Data Processing Pipelines. InHigh-Performance In-Memory Genome Data Analysis: How In-Memory Database Technology Accelerates Personalized Medicine 2013(pp. 55-74). Cham: Springer International Publishing.
- Fisher O, Watson N, Porcu L, Bacon D, Rigley M, Gomes RL. Cloud manufacturing as a sustainable process manufacturing route. J Manuf Syst. 2018;47:53-68.
- Setyawati E, Wijoyo H, Soeharmoko N. Relational Database Management System (RDBMS).
- Ray PP, Dash D, De D. Edge computing for Internet of Things: A survey, e-healthcare case study and future direction. J Netw Comput Appl. 2019;140:1-22.
- Bertino E, Sandhu R. Database security-concepts, approaches, and challenges. IEEE T Depend Secure. 2005;2(1):2-19.
Citation: Kumar M, Patel P, Kurmi BD (2025) Adoption of Pharma 4.0 from Industry 4.0 and Automation of Quality Assurance. Adv Pharmacoepidemiol Drug Saf. 14:379.
Copyright: © 2025 Kumar M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.