Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 13, Issue 4
Acetyl Amino Acids Prevent Caspase 9-mediated Apoptosis Induced by Octanoate in Mesenchymal Stem Cells
Osamu Kamida1,2*, Shuo-Hao Huang2, Akira Kawaguchi1 and Takahisa Shimizu22Graduate School of Pharmacy, International University of Health and Welfare, Otawara, Tochigi 324-8501, Japan
Received: 02-Jun-2023, Manuscript No. JSCRT-23-21647; Editor assigned: 05-Jun-2023, Pre QC No. JSCRT-23-21647(PQ); Reviewed: 26-Jun-2023, QC No. JSCRT-23-21647; Revised: 28-Jun-2023, Manuscript No. JSCRT-23-21647(R); Published: 18-Aug-2023, DOI: 10.35248/2157-7633.23.13.602
Abstract
Raw materials affect quality of cell therapy products. Although human serum albumin products as pharmaceuticals have been reported to improve cell quality, Sodium Octanoate (SO), which is known to damage cells, is contains as an additive of human serum albumin drug products. We confirmed SO concentration-dependent decrease in cell viability and increase in apoptosis in Mesenchymal Stem Cells (MSCs). Furthermore, we detected decrease of mitochondrial transmembrane potential difference and caspase 9 activation in a dose dependent manner of SO. N-Acetyl-L-Cysteine (NAC) and N-Acetyl- L-Tryptophan (NAT) were investigated for their protective effects towards SO-induced cell death and apoptosis due to their antioxidant properties. It was identified that the SO dependent apoptosis, caspase 9 activation and cell death were suppressed by NAC or NAT. These results suggest that the SO dependent cell death on MSCs is likely due to mitochondrial dysfunction by Reactive Oxygen Species (ROS) generation or cytoplasmic release of cytochrome c, and the acetyl amino acids play a critical role in inhibiting the SO dependent cytotoxicity on MSCs. Our research indicated that inhibitory effects of acetyl amino acids may contribute to the process development in cell therapy field.
Keywords
Sodium octanoate (SO), Mesenchymal Stem Cells (MSCs), Dental Pulp-Derived Stem Cells (DPSCs), Storage stability, Cell death, Apoptosis, Mitochondrial transmembrane potential difference (ΔΨm), Caspase 9
Introduction
In cell therapy field, it is necessary to deliver regenerative medicines as living cells into medical institutions, and the products are required to be transported in good quality via controlled temperature, qualified delivery time, and allowed vibration resistance. Therefore, optimization for cryopreservation process is one of the most applicable solutions for inhibiting the thermal and physical damages [1-3]. Cryopreservation process has some hurdles to maintain high quality of cell therapy products, and for example, suppression of ice crystal formation is required for cell freezing, because it is one of the most critical matters in cryopreservation process [4]. Materials used for conducting cryopreservation must be safe to be administered into patients while maintaining the product quality specification. To reduce deterioration of quality for cell therapy products, cryoprotectants or stabilizing substances, such as Human Serum Albumin (HSA), are utilized in manufacturing process development. HSA has a characteristic for stabilizing the quality of cell therapy products [5-7], and HSA drug products as pharmaceuticals are utilized as excipients of the products in clinical applications [8-10]. HSA drug products contain excipients such as Sodium Octanoate (SO) to stabilize the structure of albumin during viral inactivation in the manufacturing process [11-13]. SO has also been found to enhance the thermal stabilization of HSA by binding to HSA. On the other hand, in the presence of 0.5 mM-0.6 mM SO, the proliferation of MSCs is reduced by about 50% [14]. Also, SO has a function effects on adipocyte differentiation in vitro [15,16], so caution should be required in its usage during long-term culture of MSCs, for instance. Even though SO has effects on cell growth and death, detailed information regarding its effect is not currently available for MSCs. As SO contained in HSA drug products is quite conceivable to affect cell quality, the effects on the target cells need to be evaluated when using the HSA drug products during cell therapy product manufacturing, such as cell culture, cell harvest, buffer exchange or cryopreservation. On cell therapy product manufacturing, buffer exchange process is critical step in cell culture, harvest, and formulation. Formulation process tends to expose cells to drastic environmental changes from the culture process because cell culture medium is set for an appropriate condition for cell environment, whereas formulation requires solution containing simpler components to minimize human safety risk. There are some cases that HSA drug products is used during buffer exchange process to prevent quality deterioration, however it could take long time for multiple buffer exchange process to reduce process derived impurities. So that, the quality risk may increase due to long-term exposure of SO contained in HSA drug products.
On the other hand, it is poorly understood how HSA drug products containing SO should be appropriately used in the manufacturing process on cell therapy products. Understanding the effects of SO on stem cells will enable the appropriate usage of HSA drug products during manufacturing process and may improve the quality of cell products. The advantage of HSA drug products as a cryoprotectant or a stabilizer for cell products is supported in marketed regenerative medicines such as TEMCELL®HS Inj and Alofisel® [17-19]. On the other hand, some HSA drug products manufactured by using recombinant HSA protein do not contain additional sodium octanoate, but there are concerns regarding high manufacturing costs and the immunogenicity derived from the host cells used to produce the proteins [20,21]. Therefore, these problems suggest that there are some advantages of using HSA drug products from blood donation for regenerative medicine field. One of the most critical steps for assuring the quality of cell products are considered to be the process harvesting and concentrating viable cells after cell culture and replacing them with various buffers [22]. The cell viability, which is a critical quality attribute for the drug product specification in cell therapy field [23,24], is also measured in the process where cells adherent to the culture device are stored as a cell suspension after treatment with a cell dissociation solution such as trypsin [25]. Therefore, some processing time such as process working time, holding time need to be set to construct appropriate manufacturing process. HSA drug products are utilized for cell therapy products, and furthermore HSA has cytoprotective effects, however there is the report that blood derived HSA drug product decreased MSCs quality on storage stability evaluation [26]. This controversial evidence implies that component-based research for quality characterization has not been fully conducted for the cell therapy products development regarding to HSA drug products as well as their excipients, such as SO.
There is great uncertainty about why SO affect cell viability and how to inhibit them. Additionally, in the filling process, drug substances may be dispensed into containers, such as vials and bags, and it is assumed that some processing time is required for this filling process as well. Furthermore, the time required from cell harvest process to cryopreservation process depends on the manufacturing scale, but holding time is needed in consideration of manufacturing deviations, etc. Therefore, the storage stability of cell quality should be evaluated in hours to days. Identifying the storage stability of cells for SO, which is included as an excipient in HSA drug products, from the viewpoint of the manufacturing process of cellular products is very meaningful information for cell therapy research and engineering. Moreover, identifying the influence of cell death during storage and understanding the mechanism of cell death lead to a valuable and informative knowledge for problem-solving regarding to inhibit cell death. Furthermore, investigating the mechanism of cell death may play a significant role of manufacturing high-quality cell products.
Therefore, the objectives of this research are to investigate effect on cell storage stability in the presence of SO, and to identify the mechanism of cytotoxicity using MSCs that expect various pharmacological efficacies as cell therapy products. In this study, we identified that there is the cytotoxic effect, which means cell death, apoptosis, mitochondrial dysfunction, and caspase 9 activation, on cell storage stability in the presence of SO using human Dental Pulp Stem Cells (hDPSCs) as the model of MSCs. Furthermore, we identified that acetyl amino acids suppress SO dependent cytotoxicity.
Materials and Methods
Cells and reagents
Human dental pulp-derived mesenchymal stem cells (hDPSCs, Product Code: PT-5025, Lot. 0000611163) was purchased from Lonza. For the reagents used for cell culture and cell death evaluation, the buyer is shown below. MEMα, (GlutaMAX Supplement, no nucleosides), FBS (Fetal Bovine Serum), Anti-Anti (100X) (Antibiotic-Antimycotic), TrypLE Express, DPBS (Dulbecco’s Phosphate Buffered Saline), Trypan Blue Stain 0.4%, SYTOX Red Dead Cell Stain for 633 nm or 635 nm excitation (SYTOX), and Ultra-Pure Distilled Water (water) were purchased from Thermo Fisher Scientific. Sodium octanoate, DMSO (Culture Sure DMSO), N-Acetyl-L-tryptophan, were purchased from Fujifilm Wako Pure Chemical Industries, Ltd. Albumin solution human (30% Human serum albumin (30% HSA)), N-Acetyl-L-cysteine were purchased from Sigma Aldrich. Annexin V-FITC, Annexin V Binding Buffer, 10X concentrate (10 × Binding Buffer) were purchased from BECTON DICKINSON (BD Pharmingen). Via 1-Cassette was purchased from Chemometec, CELLBANKER 1 from Nippon Whole Pharmaceutical Industry Co., Ltd., Caspase 9 (active) FITC Staining Kit from Abcam, and JC-1 MitoMP Detection Kit from Dojindo Chemical Research Institute.
hDPSCs Culture
DPSCs were cultured in MEMα containing 10% FBS and 1% Antibiotic-Antimycotic (growth media) at 37ºC with 5% CO2 incubator. hDPSCs were cultured in their growth media until they reached 80%-90% confluence. The cells were washed once with DPBS, and DPBS was replaced with DPBS (0.1% HSA) before each assay as followed:
Cell viability study: To determine cell viability, cultured hDPSCs were resuspended with DPBS (0.1% HSA). The final cell concentration was set to 5 × 105 cells/mL and the cells were treated with the 0 mM, 1 mM, 2 mM, 4 mM, 8 mM, or 16 mM SO for 24 or 48 hours at 4°C. The cells were washed twice with DPBS (0.1% HSA) and the cell viability was measured using NC-200 (Chemometec).
Flow cytometric detection of apoptosis cells: To measure apoptosis, cultured hDPSCs were resuspended with DPBS (0.1% HSA). The final cell concentration was set to 5 ×105 cells/mL and the cells were treated with the 0 mM, 1 mM, 2 mM, 4 mM, 8 mM, or 16 mM SO for 6 hours at 4°C. The cells were washed once with 1 × Binding Buffer, which was prepared by diluting Annexin V Binding Buffer (10X concentrate) and the cells were stained with 1 × Binding Buffer containing Annexin V-FITC and SYTOX for 20 min at room temperature. After staining, the cells were washed twice with 1 × Binding Buffer and were measured using flow cytometer (CytoFLEX S). After the measurement, the analysis was carried out with FlowJo software.
Flow cytometric detection of mitochondrial membrane potential variation with JC-1 staining: To detect mitochondrial membrane potential difference (ΔΨm), cultured hDPSCs were resuspended with DPBS (0.1% HSA). The final cell concentration was set to 5-7.5 × 105 cells/mL and the cells were treated with the 0 mM, 2 mM, 4 mM, 8 mM, or 16 mM SO for 6 hours at 4°C. The cells were washed once with DPBS (0.1% HSA) and the cells were stained with DPBS (0.1% HSA) containing 100 µM of JC-1 (JC-1 Mito MP Detection Kit from Donindo Chemical Research Institute) for 20 minutes at 37°C. After staining, the cells were washed once with DPBS (0.1% HSA), were suspended with 1 × Imaging Buffer, which was prepared by diluting 10 × Imaging Buffer from JC-1 Mito MP Detection Kit, and were measured using flow cytometer (CytoFLEX S).After the measurement, the analysis was carried out with FlowJo software.
Flow cytometric detection of caspase 9 activity: To evaluate caspase 9 activity, cultured hDPSCs were resuspended with DPBS (0.1% HSA). The final cell concentration was set to 5 × 105 cells/mL and the cells were treated with the 0 mM, 2 mM, 4 mM, 8 mM, or 16 mM SO for 3 hours at 4°C. The cells were washed once with DPBS (0.1% HSA) and the cells were stained with DPBS (0.1% HSA) containing 500-fold diluted caspase 9 substrate (Caspase 9 (active) FITC Staining Kit from Abcam) and1000-fold diluted SYTOX for 20 minutes at 37°C. After staining, the cells were washed twice with DPBS (0.1% HSA) and were measured using flow cytometer (CytoFLEX S). After the measurement, the analysis was carried out with FlowJo software.
Effects of N-Acetyl-L-Cysteine and N-Acetyl-L-Tryptophan on storage stability
To evaluate the effect of NAC or NAT, hDPSCs were added with/without 5 mM NAC or NAT at the same time for being treated with SO. Apoptosis detection, caspase 9 activity, and cell viability were conducted by the methods described above.
Statistical analysis
The analysis was performed with the statistical analysis software JMP (SAS Institute, version 14.0.0). Cellular viability test, mitochondrial membrane potential detection test by JC-1 stain and caspase 9 activity test were analyzed by Dunnett’s test. Caspase 9 activity test, Annexin V stain apoptosis detection test and cell viability test, which were performed as the assessment of the effect of N-Acetyl-L- cysteine or N-Acetyl-L-tryptophan addition on the storage stability of SO reactive cells, were analyzed by Tukey-Kramer HSD test.
Results
Cell viability study
To investigate effect of octanoate on cell viability, hDPSCs were resuspended in DPBS (0.1% HSA) with different concentration of SO, and cell viability by different SO concentrations were evaluated after storing the cells for 24 hours and 48 hours under 4°C condition. In the presence of 0 mM, 1 mM, 2 mM, 4 mM, 8 mM, and 16 mM, mean cell viabilities were 87.8%, 90.8%, 80.7%, 56.6%, 39.7%, and 38.3% after 24 hours of storage, respectively. The viability for cells treated with 8 mM or 16 mM SO was significantly reduced compared to that in the absence of SO (Figure 1A). After 48 hours of storage, mean cell viabilities were 86.8%, 58.8%, 25.8%, 17.5%, 13.5%, and 13.9%, respectively. A significant decrease in viability was detected for all SO addition groups (Figure 1B). Cell viability was maintained over 80% in 0 mM condition throughout the experimental duration. From these results, SO concentration- dependent and time-dependent cell death occurred when the cell suspensions were stored at 4°C.
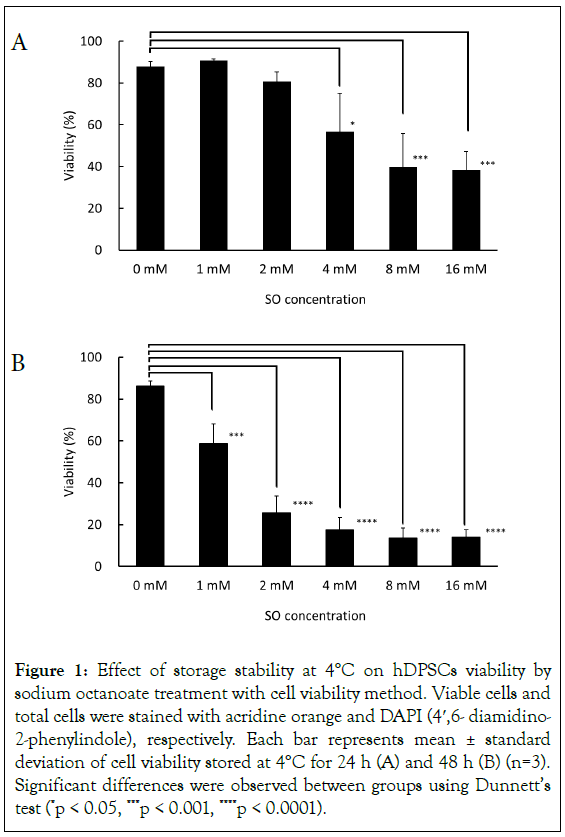
Figure 1: Effect of storage stability at 4°C on hDPSCs viability by sodium octanoate treatment with cell viability method. Viable cells and total cells were stained with acridine orange and DAPI (4′,6- diamidino- 2-phenylindole), respectively. Each bar represents mean ± standard deviation of cell viability stored at 4°C for 24 h (A) and 48 h (B) (n=3). Significant differences were observed between groups using Dunnett’s test (*p < 0.05, ***p < 0.001, ****p < 0.0001).
Apoptosis detection
In order to determine whether SO-triggered cell death is attributed to the apoptosis of hDPSCs, the degree of apoptosis was measured after storing the cells for 6 hours at 4°C in the presence of 0 mM, 1 mM, 2 mM, 4 mM, 8 mM, and 16 mM by flow-cytometry using Annexin V/SYTOX double-staining. From SO concentrations more than 2 mM, the cell viability decreased, and apoptotic cells were detected (Figure 2). Moreover, higher SO concentration was found to increase the population of late apoptotic cells.
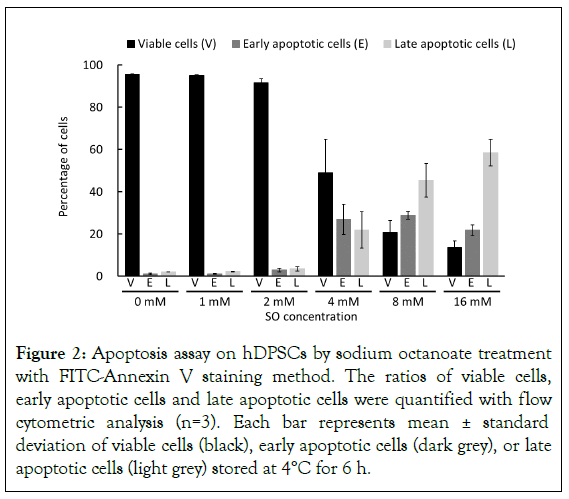
Figure 2: Apoptosis assay on hDPSCs by sodium octanoate treatment with FITC-Annexin V staining method. The ratios of viable cells, early apoptotic cells and late apoptotic cells were quantified with flow cytometric analysis (n=3). Each bar represents mean ± standard deviation of viable cells (black), early apoptotic cells (dark grey), or late apoptotic cells (light grey) stored at 4°C for 6 h.
Measurement of mitochondrial membrane potential difference by JC-1 staining
To investigate the reason why apoptosis occurred in hDPSCs treated by SO, ΔΨm as mitochondrial function was evaluated. After storing cells for 6 hours at 4°C in the presence of 0 mM, 2 mM, 4 mM, 8 mM, and 16 mM SO, JC-1 fluorescence dye, which has dual fluorescent property of either green fluorescent monomer as depolarization or red fluorescent aggregates as hyperpolarization, was used for monitoring the ΔΨm by flow cytometry. The mean ratio of aggregates/monomer was 10.1%, 10.2%, 6.3%, 1.4%, and 0.9% after 6 hours of storage, respectively. A significant decrease in the membrane potential was detected by SO at concentrations more than 2 mM (Figure 3). Additionally, SO concentration-dependent decrease of ΔΨm was detected.
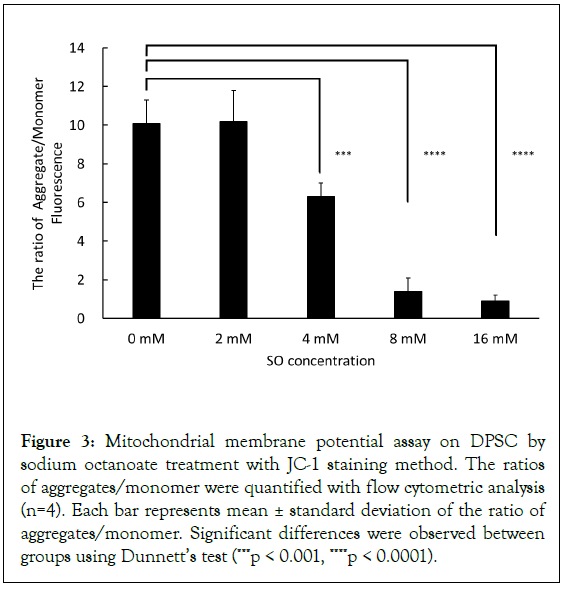
Figure 3: Mitochondrial membrane potential assay on DPSC by sodium octanoate treatment with JC-1 staining method. The ratios of aggregates/monomer were quantified with flow cytometric analysis (n=4). Each bar represents mean ± standard deviation of the ratio of aggregates/monomer. Significant differences were observed between groups using Dunnett’s test (***p < 0.001, ****p < 0.0001).
Caspase 9 activity
Mitochondrial dysfunction, such as ROS generation and cytoplasmic release induction of cytochrome c, leads to caspase 9 activation. To confirm that mitochondrial dysfunction by SO is related to apoptosis on hDPSCs, caspase 9 activity was evaluated, storing for 3 hours at 4°C in the presence of 0 mM, 2 mM, 4 mM, 8 mM, and 16 mM SO. The mean ratio of caspase 9 positive cells was 7.0%, 7.3%, 13.4%, 60.0%, and 75.4%, respectively. Significant increase in caspase 9 activity was detected with SO at concentrations of 8 mM and 16 mM (Figure 4). A mild increase in caspase 9 activity was observed at 4 mM SO, although the difference from the control sample was not significant.
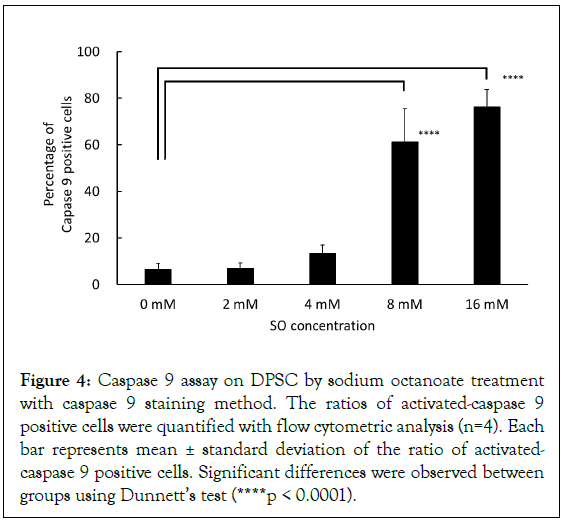
Figure 4: Caspase 9 assay on DPSC by sodium octanoate treatment with caspase 9 staining method. The ratios of activated-caspase 9 positive cells were quantified with flow cytometric analysis (n=4). Each bar represents mean ± standard deviation of the ratio of activated- caspase 9 positive cells. Significant differences were observed between groups using Dunnett’s test (****p < 0.0001).
Evaluation on effect of acetyl amino acids on the storage stability of SO-reacted cells
To confirm that anti-oxidative effect by acetyl amino acids on hDPSCs, NAC or NAT were added in the condition of DPSCs storage stability. Preventive effect of the acetyl amino acid against apoptosis by SO was evaluated, storing for 6 hours at 4°C in the presence of 0 mM, 2 mM, 4 mM, 8 mM, and 16 mM SO, and with or without 5 mM NAC and NAT. The addition of NAC and NAT significantly suppressed the generation of early apoptosis- positive cells at concentrations of 8 mM and 16 mM SO (Figure 5A). Additionally, the increase of late apoptosis-positive cells was observed due to SO concentration dependent manner and were significantly suppressed at concentrations of 8 mM and 16 mM SO with NAC and NAT (Figure 5B).
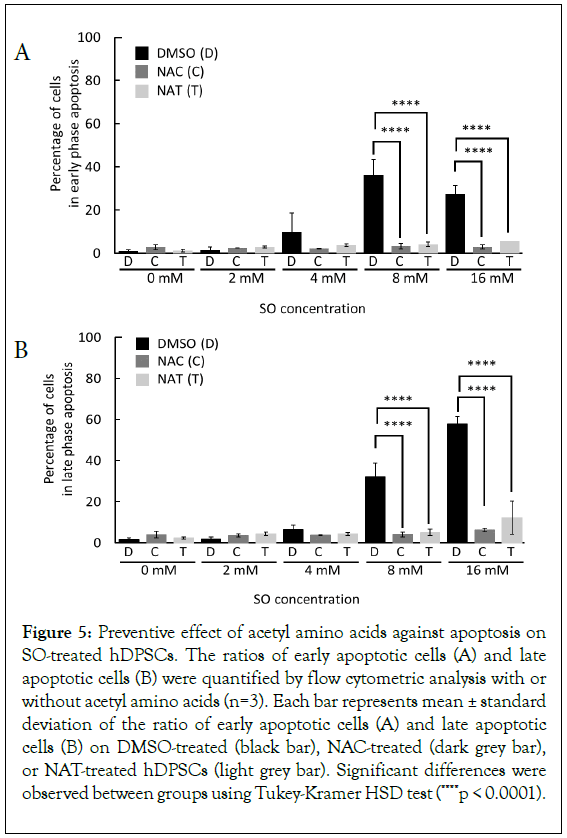
Figure 5: Preventive effect of acetyl amino acids against apoptosis on SO-treated hDPSCs. The ratios of early apoptotic cells (A) and late apoptotic cells (B) were quantified by flow cytometric analysis with or without acetyl amino acids (n=3). Each bar represents mean ± standard deviation of the ratio of early apoptotic cells (A) and late apoptotic cells (B) on DMSO-treated (black bar), NAC-treated (dark grey bar), or NAT-treated hDPSCs (light grey bar). Significant differences were observed between groups using Tukey-Kramer HSD test (****p < 0.0001).
Next, to verify inhibitory effect of acetyl amino acids against apoptosis on a molecular basis, effect of acetyl amino acids was evaluated on caspase 9 activity when stored for 3 hours at 4°C in the presence of 8 mM SO, and at the same time in the presence or absence of 5 mM NAC or NAT. We identified that the addition of NAC or NAT significantly suppressed SO-dependent activation of caspase 9 (Figures 6A and 6B). Moreover, these suppressive effects led to similar caspase 9 activation levels to controls (SO (-) groups).
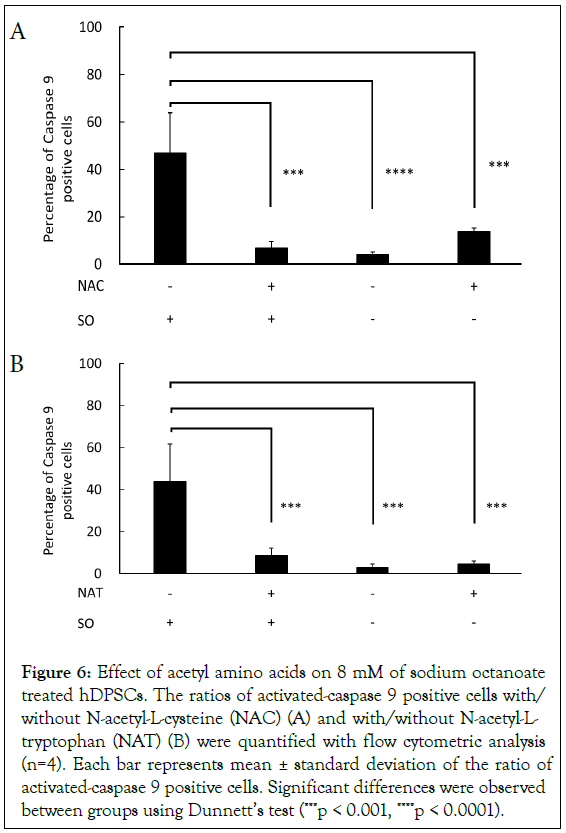
Figure 6: Effect of acetyl amino acids on 8 mM of sodium octanoate treated hDPSCs. The ratios of activated-caspase 9 positive cells with/ without N-acetyl-L-cysteine (NAC) (A) and with/without N-acetyl-L- tryptophan (NAT) (B) were quantified with flow cytometric analysis (n=4). Each bar represents mean ± standard deviation of the ratio of activated-caspase 9 positive cells. Significant differences were observed between groups using Dunnett’s test (***p < 0.001, ****p < 0.0001).
Finally, to investigate that the preventive activity of acetyl amino acid affects whole cell function, cell viability was evaluated storing for 24 hours and 48 hours at 4°C in the presence of 0 mM, 2 mM, 4 mM, 8 mM, and 16 mM SO, and with or without 5 mM NAC and NAT. After 24 hours of storage, the cell death detected with 8 mM and 16 mM SO was significantly suppressed by the addition of NAC or NAT (Figure 7A). Additionally, after 48 hours of storage, cell death detected in the presence of SO was significantly suppressed by the addition of NAC or NAT (Figure 7B). The addition of NAC or NAT significantly increased cell viability compared with treatment by 16 mM SO, however these protective effects on cell viability did not lead to fully recover cell viability in the absence of SO (Figure 7B, 0 mM SO vs. 16 mM SO treated by NAC; P<0.05, 0 mM SO vs. 16 mM SO treated by NAT; P<0.01).
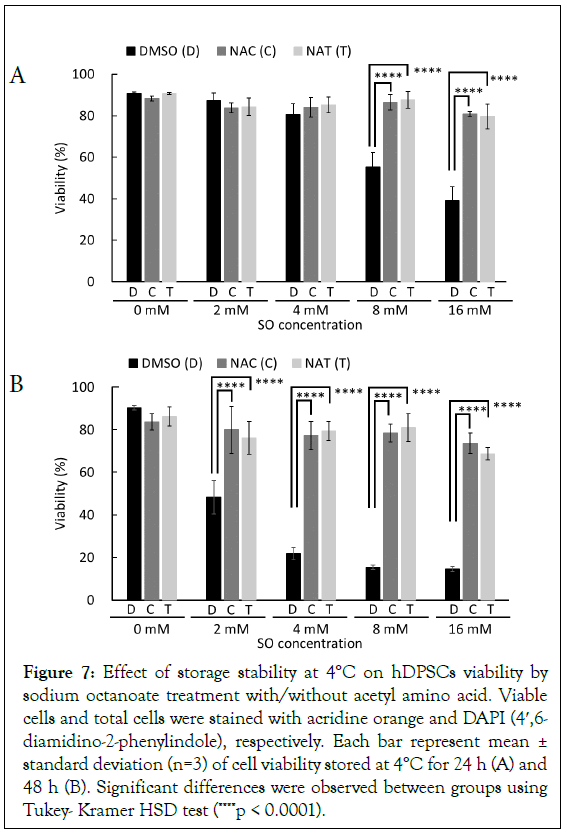
Figure 7: Effect of storage stability at 4°C on hDPSCs viability by sodium octanoate treatment with/without acetyl amino acid. Viable cells and total cells were stained with acridine orange and DAPI (4′,6- diamidino-2-phenylindole), respectively. Each bar represent mean ± standard deviation (n=3) of cell viability stored at 4°C for 24 h (A) and 48 h (B). Significant differences were observed between groups using Tukey- Kramer HSD test (****p < 0.0001).
Discussion
We conducted this research in order to clarify the effect of SO on cell storage stability, and to identify the mechanism of SO-induced cytotoxicity using hDPSCs as the model of MSCs that are expected to have various pharmacological efficacies in cell therapy fields. In this study, we evaluated whether SO dependent cytotoxicity on hDPSCs during the hypothermic storage and acetyl amino acids can rescue SO-induced cell damage. The results from cell viability, apoptosis, mitochondrial transmembrane potential difference (ΔΨm), and caspase 9 activity suggested that the SO at high concentration attenuate the function of mitochondria by suppressing the ΔΨm, and consequently activates caspase 9, leading to apoptosis and cell death. Caspase 9 is activated by mitochondrial dysfunction, ROS generation and cytoplasmic release induction of cytochrome c [27-28]. It has been reported that NAC and NAT could inhibit ROS generation and cytoplasmic release of cytochrome c, respectively [29-30]. We found that the addition of NAC and NAT suppressed caspase 9 activation, apoptosis, and cell death induced by SO in hDPSCs. The results supported that SO has a characteristic of inhibiting mitochondrial function and the induced mitochondrial dysfunction can be rescued by the antioxidant effects of NAC and NAT. Juan et al. reported that FDA and EMA specified the minimal cell viability for MSC-based cell therapy products as 70% and 80%, respectively [23]. We consider that these criterions help it prove the justification that cell viability was more than 80% without SO treatment shown in Figure 1 and maintained more than 70% for SO treated hDPSCs with NAC or NAT represented in Figure 7.
Our study showed that it is possible to manufacture a cell product that satisfies the specification as described above if the SO concentration is not more than 2 mM when stored within 24 hours at 4°C. HSA drug products generally contain SO at a concentration of approximately 16 mM [31]. In cell therapy manufacturing, cells could contact SO contained in HSA during buffer exchange process as well as formulation and cryopreservation process. Therefore, our study could be helpful to evaluate the effects of SO on cells during manufacturing processing. In this study, to identify the effect of SO on hDPSCs, DPBS was used as the suspension buffer due to its simple composition and 0.1% HSA was added to suppress attachment to the container during sample preparation. Acetyl tryptophan is included in HSA drug products at similar concentration as SO as an excipient [31]. For example, when HSA drug products are diluted to prepare the solution containing 5 mM acetyl tryptophan, the SO concentration contained in the diluted solution becomes 5 mM. The induction of caspase 9 activity, apoptosis, and cell death due to SO were suppressed by the addition of NAC and NAT in this study. Therefore, it is assumed that 5 mM acetyl tryptophan in the solution prepared by the diluted HSA drug products could sufficiently suppress the cell death caused by SO concentration contained in diluted HSA drug products. Reports on cell storage stability using HSA drug products have shown that cell death still occurred at HSA concentrations of 2% to 5% [26]. The estimated concentration of acetyl tryptophan calculated from Harm’s report [31] is up to 4 mM for 5% HSA drug products. This concentration of acetyl tryptophan reported by Harm et al. is close to the effective concentration of our study, while showed no protective effects. Acetyl tryptophan is an unstable amino acid and prone to degradation by light, and afterwards N-formylkynurenine, kynurenine, and quinolinic acid are generated [32,33]. Assuming this degradation sensitivity of acetyl tryptophan on MSCs storage stability with HSA drug product, acetyl tryptophan might be degraded, and the inhibitory effect of cell death caused by SO might not be sufficiently inhibited. Considering the degradation of acetyl tryptophan, additional acetyl tryptophan is more effective to rescue apoptosis and cell death of MSCs that may be caused by SO in HSA drug products. Therefore, the inhibition of cell death by NAC and NAT revealed in this experiment is significant from the viewpoint of process development for MSCs as cell therapy medicines.
It was clarified that mitochondrial function is inhibited by SO in this experiment. This result suggests that excessive amount of SO may be taken into mitochondria and mitochondrial function such as β-oxidation may have been inhibited. We proved that this decrease in the mitochondrial membrane potential was caused by sodium octanoate using JC-1 staining. Inhibition of the caspase 9 activity caused by NAC and NAT was distinctly detected at 5 mM, but at lower concentrations (e.g. 1 mM), no inhibition of caspase 9 activity could be detected (data not shown). Some reports show that NAC needs to be added at mM-order level to exert the cellular anti-oxidation, so that, the results in this study that the effective dose was 5 mM of NAC and NAT, is similar to the concentration range previously reported [34-37]. Thus, concentration of around 5 mM is considered to be required for inhibition of caspase 9 activity in hDPSCs. It was clarified that cell viability was decreased for 48 hours in the presence of 2 mM SO in Figure 1B. Additionally, it was revealed that the addition of NAC and NAT rescued the decrease in cell viability for 48 hours in the presence of 2 mM SO in Figure 7. These results indicate that long-term retention of even a small amount of sodium octanoate induces cell death, supported by the report that cell proliferation decreases by about 50% in the culture of MSCs in the presence of 0.5 mM to 0.6 mM sodium octanoate [14]. Therefore, these results suggest that the cell viability exposed by less than 1 mM SO for a longer time may be decreased and may recover cell viability by the anti-oxidative effect of NAC and NAT. It has been reported that preconditioning, such as the addition of a caspase inhibitor or under hyperoxic conditions, affects inhibition of apoptosis and cell survival [38]. The results in Figure 7b shows that the effect of NAC and NAT tend not to completely rescue SO dependent cell death compared with the negative control (SO negative sample) for 48 hours. The experimental protocol in this study does not include preconditioning of NAC and NAT. NAC and NAT were added at the same time as SO added to hDPSCs, however it is meaningful that cell death can be suppressed without preconditioning from the point of view of simpler operation in manufacturing process development.
Regarding the approach of apoptosis inhibition, there are some evidences reported as below. Apoptosis could be inhibited on ADSCs stably expressing HIF1α [39]. Down-regulation of ROS, caspase9, and caspase 3 could be observed with exogenous HIF1α, but it did not lead to rescue of mitochondrial membrane potential in the cells. Berberine activates the mitochondrial membrane potential and inhibits apoptosis of ADSCs under hypoxic conditions and no serum [40,41]. It was also reported that NAC and ascorbic acid 2-phosphate synergize to suppress mitoptosis, necrosis, and apoptosis of MSCs [42]. Furthermore, NAC has been reported to suppress the generation of ROS and apoptosis derived from FAS ligand on MSCs [43]. By conducting manufacturing using preconditioning and metabolic improvement as shown above, addition of NAC or NAT can improve the quality of manufactured cellular products. In this study, it was clarified that cell death caused by SO was suppressed in the presence of NAC or NAT, but no rescue of mitochondrial membrane potential by SO in the presence of NAC or NAT was observed (data not shown), and no molecules that directly affect SO could be identified. Addition of substances with antioxidant effects, such as berberine, which activates the membrane potential of mitochondria, and vitamin E, which suppresses the increase of lipid peroxide, may be effective for the SO dependent inhibition [44].
We utilized hDPSCs as the model of MSCs in this study. SO dependent cell death has been reported on bone marrow mesenchymal stromal cell [14], and we found similar toxic effects on hDPSCs. However, regarding other type of MSCs, evaluation of cytotoxicity including caspase 9 -mediated apoptosis as described in this study might need to be implemented because various type of MSCs can be collected from different organs of the body and the function of mitochondria may differ between each MSC types. Regarding the molecular basis relating to SO derived mitochondrial dysfunction, detailed evaluation such as metabolic flux analysis in mitochondria, will be required to expand the finding in this study. We also confirmed that either NAC or NAT significantly inhibited cytotoxicity and recovered cell functions up to the level of untreated MSCs. The additive effect of both NAC and NAT could be expected regarding inhibition of cytotoxicity; however we didn’t conduct further experiment of evaluating this additive effect because adequate prevention of cytotoxicity was detected by either NAC or NAT alone. Effect of combination with NAC and NAT as well as other antioxidants such as berberine described above may need to be evaluated with further functional analysis as mitochondrial dysfunction by SO.
Developing cell therapy products as pharmaceuticals, formulation buffer components should be considered carefully from the point of view of efficacy, safety, and quality. Basically, cells should be cultured and maintained in an appropriate medium during upstream manufacturing process, and for that reason it is very difficult to select a suitable solution to be used for formulation process to maintain the cell function and viability. Regarding development of formulation process, not only formulation buffer selection, but also determination of buffer exchange solution after cell harvest should be required, because formulation process is a drastic exchange from nutrient rich environment like an appropriate medium to simple component in order to minimize human safety risk. Based on the various evidences described above, utilization of HSA drug products is considered to be significant from the viewpoint of development of cell therapy products, because HSA prevents cell loss, protects cells during freezing, and also protects cells from adsorption to the container. However, the potentially detrimental effects of SO included in HSA drug products must be considered carefully because of the results in this study. Adding either NAC or NAT can not only ameliorate the SO-induced apoptosis and cell death, but also contribute to development of formulation buffer solution of cell therapy product or development of manufacturing process such as formulation development.
Conclusion
Our results suggest that the SO dependent cell death on MSCs is likely due to mitochondrial dysfunction and caspase 9-mediated apoptosis. This study clarified that SO triggered caspase 9 activation dependent on mitochondrial dysfunction by ROS generation or cytoplasmic release of cytochrome c, and the acetyl amino acids play a critical role in inhibiting the SO dependent cytotoxicity on MSCs. Our research indicated that inhibitory effects of acetyl amino acids may contribute to the process development in cell therapy field. We also provide usefulness for improving manufacturability and quality of cell therapy products.
Acknowledgment
We thank Masayuki Yabuta and Uichi Koshimizu for administrative support for this research.
References
- Zhurova M, Woods EJ, Acker JP. Intracellular ice formation in confluent monolayers of human dental stem cells and membrane damage. Cryobiology. 2010;61(1):133-141.
[Crossref] [Google Scholar] [PubMed]
- Renzi S, Lombardo T, Dotti S, Dessì SS, De Blasio P, Ferrari M. Mesenchymal stromal cell cryopreservation. Biopreserv Biobank. 2012;10(3):276-281.
[Crossref] [Google Scholar] [PubMed]
- Jang TH, Park SC, Yang JH, Kim JY, Seok JH, Park US, et.al. Cryopreservation and its clinical applications. Integr Med Res. 2017;6(1):12-18.
[Crossref] [Google Scholar] [PubMed]
- Asghar W, El Assal R, Shafiee H, Anchan RM, Demirci U. Preserving human cells for regenerative, reproductive, and transfusion medicine. Biotechnol J. 2014;9(7):895-903.
[Crossref] [Google Scholar] [PubMed]
- Burnham RE, Tope D, Branella G, Williams E, Doering CB, Spencer HT. Human serum albumin and chromatin condensation rescue ex vivo expanded γδ T cells from the effects of cryopreservation. Cryobiology. 2021;99:78-87.
[Crossref] [Google Scholar] [PubMed]
- Richards M, Fong CY, Tan S, Chan WK, Bongso A. An efficient and safe xeno-free cryopreservation method for the storage of human embryonic stem cells. Stem Cells. 2004;22(5):779-789.
[Crossref] [Google Scholar] [PubMed]
- Bamba K, Ozawa M, Tamai M, Shimbo E, Shindo H, Ota T, et.al. Human serum albumin-containing xeno-free freezing medium optimized for regenerative medicine. Translational and Regulatory Sciences. 2021;3(3):77-82.
- Li RU, Johnson R, Yu G, McKenna DH, Hubel A. Preservation of cell-based immunotherapies for clinical trials. Cytotherapy. 2019;21(9):943-957.
[Crossref] [Google Scholar] [PubMed]
- Shahin H, Elmasry M, Steinvall I, Markland K, Blomberg P, Sjöberg F, et.al. Human serum albumin as a clinically accepted cell carrier solution for skin regenerative application. Sci Rep. 2020 Sep;10(1):14486.
[Crossref] [Google Scholar] [PubMed]
- Yuan Z, Lourenco SD, Sage EK, Kolluri KK, Lowdell MW, Janes SM. Cryopreservation of human mesenchymal stromal cells expressing TRAIL for human anti-cancer therapy. Cytotherapy. 2016;18(7):860-869.
[Crossref] [Google Scholar] [PubMed]
- Faroongsarng D, Kongprasertkit J. The role of caprylate ligand ion on the stabilization of human serum albumin. AAPS PharmSciTech. 2014;15(2):465-471.
[Crossref] [Google Scholar] [PubMed]
- Kouno Y, Anraku M, Yamasaki K, Okayama Y, Iohara D, Ishima Y, et.al. N-acetyl-l-methionine is a superior protectant of human serum albumin against photo-oxidation and reactive oxygen species compared to N-acetyl-l-tryptophan. Biochim Biophys. 2014;1840(9):2806-2812.
[Crossref] [Google Scholar] [PubMed]
- Anraku M, Tsurusaki Y, Watanabe H, Maruyama T, Kragh-Hansen U, Otagiri M. Stabilizing mechanisms in commercial albumin preparations: octanoate and N-acetyl-L-tryptophanate protect human serum albumin against heat and oxidative stress. Biochim Biophys Acta. 2004;1702(1):9-17.
[Crossref] [Google Scholar] [PubMed]
- Wong WW, MacKenzie AD, Nelson VJ, Faed JM, Turner PR. Octanoate in human albumin preparations is detrimental to mesenchymal stromal cell culture. Stem Cells Int. 2015; 2015.1-8.
[Crossref] [Google Scholar] [PubMed]
- Takenouchi T, Takayama Y, Takezawa T. Co-treatment with dexamethasone and octanoate induces adipogenesis in 3T3-L1 cells. Cell Biol Int. 2004;28(3):209-216.
[Crossref] [Google Scholar] [PubMed]
- Suzuki S, Suzuki M, Sembon S, Fuchimoto D, Onishi A. Characterization of actions of octanoate on porcine preadipocytes and adipocytes differentiated in vitro. Biochem Biophys Res Commun. 2013;432(1):92-98.
[Crossref] [Google Scholar] [PubMed]
- Murata M, Teshima T. Treatment of steroid-refractory acute graft-versus-host disease using commercial mesenchymal stem cell products. Front Immunol. 2021;12:724380.
[Crossref] [Google Scholar] [PubMed]
- Scott LJ. Darvadstrocel: a review in treatment-refractory complex perianal fistulas in Crohn’s disease. BioDrugs. 2018;32(6):627-634.
[Crossref] [Google Scholar] [PubMed]
- European Medicines Agency: Public assessment report for Alofisel. 2018.
- Vanderlaan M, Zhu-Shimoni J, Lin S, Gunawan F, Waerner T, Van Cott KE. Experience with host cell protein impurities in biopharmaceuticals. Biotechnol Prog. 2018;34(4):828-837.
[Crossref] [Google Scholar] [PubMed]
- Wang F, Li X, Swanson M, Guetschow E, Winston M, Smith JP, et.al. Holistic analytical characterization and risk assessment of residual host cell protein impurities in an active pharmaceutical ingredient synthesized by biocatalysts. Biotechnol Bioeng. 2022;119(8):2088-2104.
[Crossref] [Google Scholar] [PubMed]
- Lechanteur C, Briquet A, Bettonville V, Baudoux E, Beguin Y. MSC manufacturing for academic clinical trials: from a clinical-grade to a full GMP-compliant process. Cells. 2021;10(6):1320.
[Crossref] [Google Scholar] [PubMed]
- Guadix JA, López-Beas J, Clares B, Soriano-Ruiz JL, Zugaza JL, Gálvez-Martín P. Principal criteria for evaluating the quality, safety and efficacy of hMSC-based products in clinical practice: current approaches and challenges. Pharmaceutics. 2019;11(11):552.
[Crossref] [Google Scholar] [PubMed]
- Radrizzani M, Soncin S, Bolis S, Lo Cicero V, Andriolo G, Turchetto L. Quality control assays for clinical-grade human mesenchymal stromal cells: validation strategy. Mesenchymal Stem Cells. 2016:339-356.
[Crossref] [Google Scholar] [PubMed]
- Weber C, Pohl S, Pörtner R, Wallrapp C, Kassem M, Geigle P, et.al. Expansion and harvesting of hMSC-TERT. Open Biomed Eng J. 2007;1:38-46.
[Crossref] [Google Scholar] [PubMed]
- Mirabel C, Puente-Massaguer E, del Mazo-Barbara A, Reyes B, Morton P, Gòdia F, et.al. Stability enhancement of clinical grade multipotent mesenchymal stromal cell-based products. J Transl Med. 2018;16(1) 1659-1664.
[Crossref] [Google Scholar] [PubMed]
- Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13(9):1423-1433.
[Crossref] [Google Scholar] [PubMed]
- Chen Q, Chai YC, Mazumder S, Jiang C, Macklis RM, Chisolm GM, et.al. The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction. Cell Death Differ. 2003 ;10(3):323-334.
[Crossref] [Google Scholar] [PubMed]
- Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60(1):6-20.
[Crossref] [Google Scholar] [PubMed]
- Sirianni AC, Jiang J, Zeng J, Mao LL, Zhou S, Sugarbaker P, et.al. N-acetyl-l-tryptophan, but not N-acetyl-d-tryptophan, rescues neuronal cell death in models of amyotrophic lateral sclerosis. J Neurochem. 2015;134(5):956-968.
[Crossref] [Google Scholar] [PubMed]
- Harm S, Schildböck C, Hartmann J. Removal of stabilizers from human serum albumin by adsorbents and dialysis used in blood purification. PLoS One. 2018;13(1):e0191741.
[Crossref] [Google Scholar] [PubMed]
- Ehrenshaft M, de Oliveira Silva S, Perdivara I, Bilski P, Sik RH, Chignell CF ,et.al. Immunological detection of N-formylkynurenine in oxidized proteins. Free Radic Biol Med. 2009;46(9):1260-1266.
[Crossref] [Google Scholar] [PubMed]
- La Cruz VP, Carrillo-Mora P, Santamaría A. Quinolinic acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int J Tryptophan Res.2012;5:1-8.
[Crossref] [Google Scholar] [PubMed]
- Berniakovich I, Laricchia-Robbio L, Belmonte JC. N-acetylcysteine protects induced pluripotent stem cells from in vitro stress: impact on differentiation outcome. Int J Dev Biol. 2012;56(9):729-735.
[Crossref] [Google Scholar] [PubMed]
- Dang S, Yu ZM, Zhang CY, Zheng J, Li KL, Wu Y, et.al. Autophagy promotes apoptosis of mesenchymal stem cells under inflammatory microenvironment. Stem Cell Res Ther. 2015;6:1-9.
[Crossref] [Google Scholar] [PubMed]
- Kagihiro M, Fukumori K, Horiguchi I, Kim MH, Kino-oka M. Suppression of time-dependent decay by controlling the redox balance of human induced pluripotent stem cells suspended in a cryopreservation solution. Biochem Eng. J. 2020;155:107465.
- Liu J, Liu Q, Han J, Feng J, Guo T, Li Z, et.al. N-Acetylcysteine inhibits patulin-induced apoptosis by affecting ROS-mediated oxidative damage pathway. Toxins (Basel). 2021;13(9):595.
[Crossref] [Google Scholar] [PubMed]
- Saini U, Gumina RJ, Wolfe B, Kuppusamy ML, Kuppusamy P, Boudoulas KD. Preconditioning mesenchymal stem cells with caspase inhibition and hyperoxia prior to hypoxia exposure increases cell proliferation. J Cell Biochem. 2013;114(11):2612-2623.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Wu H, Zhou Y, Pang H, Liu Y, Oganezov G, et.al. HIF-1α inhibits mitochondria-mediated apoptosis and improves the survival of human adipose-derived stem cells in ischemic microenvironments. J Plast Reconstr Aesthet Surg. 2021;74(8):1908-1918.
[Crossref] [Google Scholar] [PubMed]
- Pang H, Zhou Y, Wang J, Wu H, Liu X, Gao F, et.al. Berberine influences the survival of fat grafting by inhibiting autophagy and apoptosis of human adipose derived mesenchymal stem cells. Drug Des Devel Ther. 2021:4795-4809.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Su X, Gao Y, Sun B, Yu Y, Wang X, et.al. Berberine protects mesenchymal stem cells against hypoxia-induced apoptosis in vitro. Biological and Pharmaceutical Bulletin. 2009 ;32(8):1335-1342.
[Crossref] [Google Scholar] [PubMed]
- Li CJ, Sun LY, Pang CY. Synergistic protection of N-acetylcysteine and ascorbic acid 2-phosphate on human mesenchymal stem cells against mitoptosis, necroptosis and apoptosis. Scientific reports. 2015;5(1):9819.
[Crossref] [Google Scholar] [PubMed]
- Rodrigues M, Turner O, Stolz D, Griffith LG, Wells A. Production of reactive oxygen species by multipotent stromal cells/mesenchymal stem cells upon exposure to fas ligand. Cell Transplant. 2012;21(10):2171-2187.
[Crossref] [Google Scholar] [PubMed]
- Ghzaiel I, Zarrouk A, Nury T, Libergoli M, Florio F, Hammouda S, et.al. Antioxidant properties and cytoprotective effect of Pistacia lentiscus L. seed oil against 7β-hydroxycholesterol-induced toxicity in C2C12 myoblasts: Reduction in oxidative stress, mitochondrial and peroxisomal dysfunctions and attenuation of cell death. Antioxidants (Basel). 2021;10(11):1772.
[Crossref] [Google Scholar] [PubMed]
Citation: Kamida O, Huang SH, Kawaguchi A, Shimizu T (2023) Acetyl Amino Acids Prevent Caspase 9-mediated Apoptosis Induced by Octanoate in Mesenchymal Stem Cells. J Stem Cell Res Ther. 13.602.
Copyright: © 2023 Kamida O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

