Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2020) Volume 0, Issue 0
A Review on Covid-19 Pandemic Outbreak
Dr. Yeshwanth Sankranthi1*, Ittedi Rajashekhar2 and Ittedi Rani32Department of Pharmacy practise,Sri Indu Institute of Pharmacy, Hyderabad, India
3Department of Pharmacy Practise, Sri Venkateshwara College of Pharmacy, Hyderabad, India
Received: 15-Nov-2020 Published: 27-Nov-2020, DOI: 10.35248/1948-5948.20.12.449
Abstract
The emergence of SARS-COV2 from Wuhan at the end of December 2019 has spread to 200 countries is the leading cause of deaths. Belonging to be β-COV with single-strand RNA attacks the human respiratory system. The COVID-19 symptoms appear after incubation. The appearance of symptoms varies depending on the age and status of the immune system. Globally, current trials are on vaccines and focused on plasma therapy with the survivor’s plasma.
Keywords
SARS-COV2; Genome; Nucleocapsid; ACE-2; Alveolar epithelial; Plasma; Social distancing; Disinfectants
Introduction
The global emergence of Severe Accurate Emergence Syndrome Corona Virus 2 (SARS-COV2) disease in December 2019, a cluster of cases of unexplained viral pneumonia was identified in Wuhan city, a metropolitan in Hubei province people republic of China (PRC) [1]. Later named as COVID-19 has caused a large outbreak globally and is leading cause of deaths worldwide [2]. The first case was reported in Wuhan seafood market where numerous types of live wild animals are sold including poultry, bats, grand hogs and snakes. According to World Health Organization (WHO) viral diseases to grow continue and emerge and respect a serious issue to public health and pose a challenge for human survival on January 30,2020. The WHO declared the outbreak of novel corona virus as public health emergency.
Several virus diseases, SARS-COV in 2002-2003, H1N1 influenza in 2009, Middle East Respiratory Syndrome Corona Virus (MERSCOV) was identified first in Saudi Arabia in 2012 and Ebola in (2014), Zika (2016) and COVID19 (2019) [2]. The corona virus poses a great challenge for health workers government and public leading for economic crisis together work will prevent the further spread.
Study Design
A scoping review was conducted by following steps:
• Identifying a clear review objective and search strategies.
• Identifying review related articles.
• Selection of review articles.
• Extraction of review information and adapting it
Literature Search Strategies
Literature for this review was identified by searching the following online databases: Google Scholar, Pubmed Central, Science Direct, The Lancet.
Data Extraction
After articles were selected, data were extracted and recorded. The extracted data includes authors name, Title of article, Name of the Journal, Year of Publication, Epidemiology, Diagnosis, Treatment.
Epidemology
The first case of unidentified viral pneumonia was reported in Wuhan city, China [3]. The origin of the disease has not been determined, as most of the confirmed cases were linked to Wuhan seafood market [4]. The virus started to spread through direct contact with infected secretions or from aerosol droplets. When these secretions reach mouth, nose, eyes of a normal individual get infected. United States of America (U.S.A) is the most affected in the record of highest number of infected cases and deaths. Later on, Britain, Italy, France, Spain are most affected due to pandemic and spread to more than 210 countries with declaration of Health Emergency.
Viral Structure and it's Bindings
The Corona Virus (COV) is a single stranded RNA virus with a diameter od 80-120nm. Corona virus belongs to the family Coronaviridae, sub family of Coronavirinae, in the order Nidovirales (Figure 1). It is genetically divided into four genera likely α-Corona virus (α-COV), β-Corona virus (β-COV), δ-Corona virus (δ-COV), γ-Corona virus (γ-COV) [5,6]. Six Corona viruses are identified previously across the globe and the SARS-COV2 is the seventh member of the Corona family belongs to β-COV [7]. The genome sequence homology of SARS-COV2 is 79% closer to SARS. The 2019 nCOV is closer to the SARS like Bat COVS (MG 772933) [8]. The Angiotensin-converting enzyme-2 (AEC-2) present in the lower respiratory tract is majorly affected in the body [9]. SARS-COV2 infects the human lung alveolar epithelial cells through receptor mediated endocytosis and causes lung infiltration [10]. The genetic sequence of SARS-COV2 is 79% similar to that of SARS like Bat COVS (MG 772933). So, SARS-COV2 is capable of using the same cell entry receptor (ACE-2) to infect humans like SARS-COV [11]. SARS-COV2 spikes bind to human ACE- 2 receptors with 10-20-fold higher affinity than the SARS-COV spike. This helps the virus to spread from human to human [12]. The SARS-COV2 virus after entering into the alveolar epithelial cells, replicates at rapid rate and triggers a strong immune response and causes respiratory distress and respiratory failure.
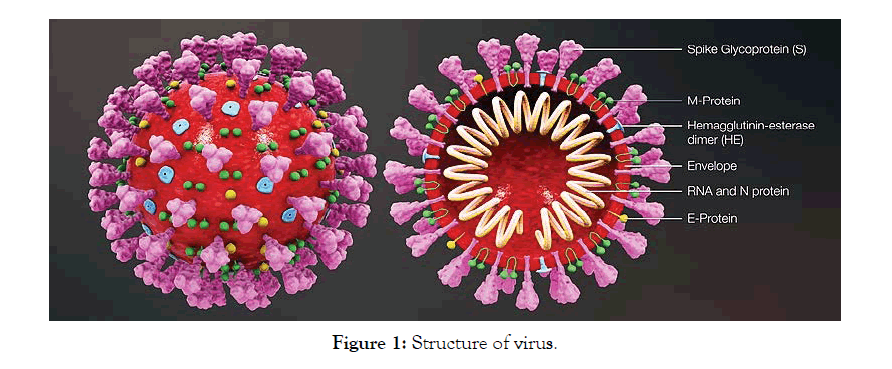
Figure 1: Structure of virus.
The electron microscope studies, across the globe has given a detailed structural information about COVID-19. The virus is covered with pointed structures that surround them like crown like Spikes (S), Glycoproteins on their Envelop (E), Membrane protein (M), and the Nucleocapsid (N) protein, which are essential for SARS-COV2 assembly and causes necessary infection [13,14].
Clinical Symptoms
The COVID-19 symptoms appear after incubation. The appearance of the symptoms vary depends on the age and status of the immune system. The conditions range from symptomatic to asymptomatic (Figurea 2, and 3). The symptoms are like
• Fever (87.9%)
• Dry cough (67.7%)
• Fatigue (38.1%)
• Sputum production (33.4%)
• Myalgia (14.8%)
• Headache (13.6%)
• Diarrhea (3.7%)
• Nausea or vomiting (5.0%)
• Hemoptysis (0.9%)
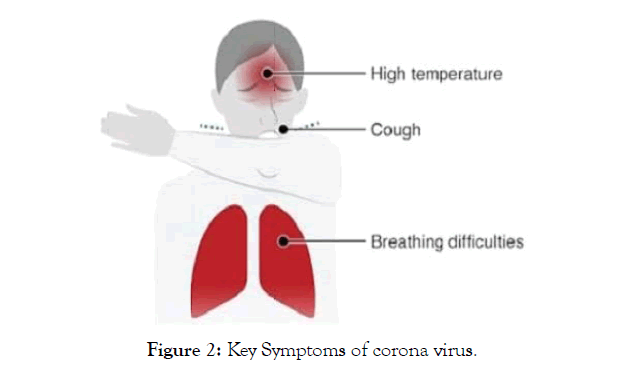
Figure 2: Key Symptoms of corona virus.
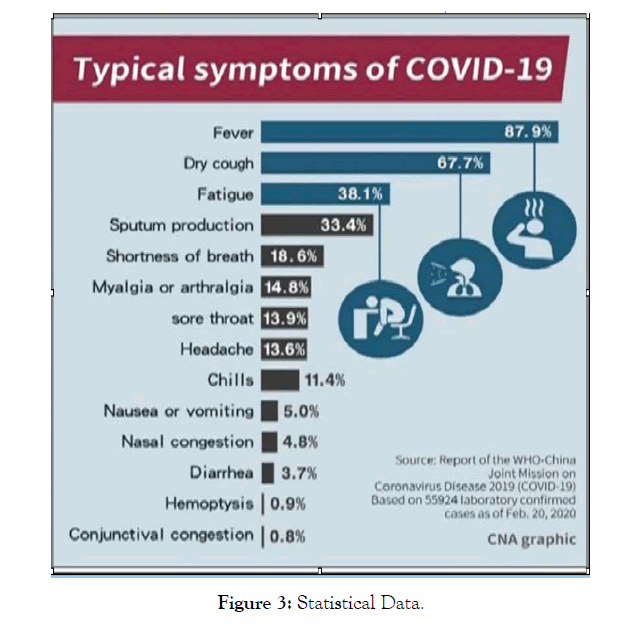
Figure 3: Statistical Data.
In some cases, it shows infiltration in the upper lobe of the lung showing symptoms of Dyspnea (18.6%) and Hypoxemia [3,15-17].
Diagnostic Techniques
The rapid and accurate detection of SARS-COV2 is necessary to control the spread of COVID-19 in the community. CT imaging of chest has shown the presence of White patches, Ground glass opacities, pleural effusion with thoracic lymphadenopathy and fibrosis of lungs [18]. The viral nucleic acid detection method is the major and confirmative method which includes the highest risk for health care workers while collecting the sample from Nasopharyngeal, Oropharyngeal swabs, stool, sputum or blood samples will be examined by RT-q PCR technique but it is long and time taking confirmative process [9]. Rapid antibody test is one of the diagnostic tools available in COVID-19 testing but the accuracy of the test has been questioned globally. Some other diagnostic techniques are available which lowers the risk to health care providers, the Rutgers Clinical Genomics Laboratory developed an RT-PCR assay (Taq path COVID-19 combo kit) that uses selfcollected saliva samples, which are quicker and less painful than the other sample collection methods [19]. Some advanced researches have shown a dual functional plasmonic biosensor combining the Plasmonic Photothermal (PPT) effects and Localized Surface Plasmon Resonance (LSPR). Sensing transduction provides an alternative diagnostic tool for detecting COVID- 19 [20].
Treatment
Globally due to the absence of vaccine and specific Anti-viral drugs to COVID-19, this pandemic is causing large number of deaths worldwide. The initial treatment has begun with some Anti-viral drugs in combination with some broad-spectrum antibiotics and supportive care for patients and later trials has begun in search of vaccine for COVID-19.
Chloroquine and Hydroxychloroquine
Chloroquine and Hydroxychloroquine (HCQ) has been used widely for the treatment of Malaria worldwide. These drugs have shown tremendous impact on COVID-19 infected patients. HCQ has shown a decrease in the viral load in infected patients. HCQ has become more potent when it is added with Azithromycin. Some adverse events like Arrhythmias has been noted in cardiac patients with COVID-19 infection with use of HCQ [21].
Anti-viral
Many Anti-viral drugs are used globally to treat SARS-COV2 along with supportive care. Remedesivir presents with antiviral activity by interfering with RNA-dependent RNA polymerase, there by inhibiting virus ability to replicate in the body. Remedesivir has originally developed to treat Ebola virus, later stages it has demonstrated with the efficacy of inhibiting SARS-COV and MERS-COV in-vitro [22]. Lopinavir/Ritonavir (LPV-r) co-formulated drug used as first line therapy in HIV. LPV-r has shown in-vitro inhibitory activity against SARS-COV during its outbreak in 2003. The other combination drugs of LPV-r and Ribavirin has shown tremendous effect in treating patients with SARS [23]. In some countries, treatment is with Human monoclonal antibody, Mesenchymal stem cells, Immuno-enhancers, Intravenous gamma globulins, NK cell therapy, Corticosteroids but there is no confirmative therapy for COVID-19.
Convalescent plasma
Globally, researchers are in hunt of vaccine for COVID-19. Apart from vaccine, research is keenly focusing on plasma therapy which is effective and successfully employed in the treatment of SARSCOV1 (2003) epidemic, H1N1 Influenza Virus (2009) pandemic and MERS-COV (2012) epidemic. The plasma therapy is collecting the plasma from the donors who survived from the attack of infectious disease [24]. The plasma trials are giving successful results and are showing a decrease in the symptoms of COVID-19.
Opinion on how to manage cases of covid-19
Prevention and control strategieS are more important. Prevention at National level, control at population level. The discovery of vaccine for COVID-19 may take a few more months. So, prevention is better way to control COVID-19.
• Identification, isolation, treatment of COVID-19 infected patients.
• Identification of primary, secondary contacts and testing.
• Identification of super spreaders.
• Importance of social distancing.
• Mask acts as a big wall for prevention of COVID-19.
• By focusing on personal hygiene and spraying surroundings with dis infectants.
• By boosting immune system (Vit-C), drinking warm water etc.
Till the approval of vaccine, prevention and control of COVID-19 at community level is biggest task.
Herd Immunity
Herd immunity is an old age concept.It is an indirect immune, when most of a population is immune to an infectious disease this provides herd immunity to those who are not immune to the disease. The pandemic SARS-COV 2 has reportedly caused 67,50,521 infected cases with 3,95,779 deaths. Numerous clinical trials are to evaluate novel vaccine and drug repurposing strategies for prevention and treatment of SARS-COV 2 Infection. However, it is unknown that these trials will produce an effective vaccine, it is unclear that how long these studies will take to establish efficacy and safety. In an optimistic estimate for any vaccine trial it takes 12- 18 months for its outcome. By threshold theorem that if immunity (vaccination) is deliverd at random and members of population mixed at random, if R0 individual is capable in transmit the infection to the individuals who gets in contacted then rate of infection would get decline if proportion of immune exceeded (R0- 1)/R0 [25-27].
As shown in Figure 4 A). Transmission over three generations after introduction into atotally susceptible population (1 case would lead to four cases and then to 16 cases).
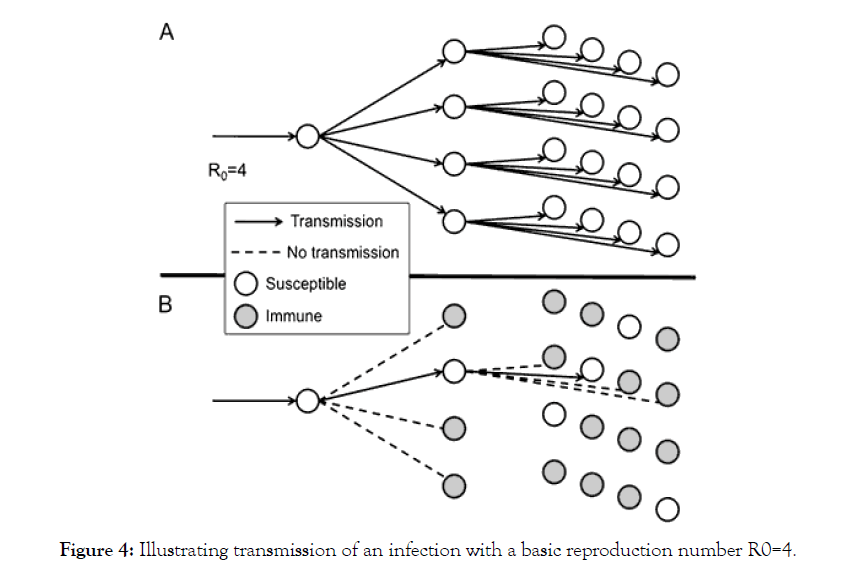
Figure 4: Illustrating transmission of an infection with a basic reproduction number R0=4.
As shown in Figure 4 B). Expected transmission if (R0-1/R0=1-1/ R0=3/4) of the population is immune.Under this circumstance, all but one of the contacts for each case s immune,and so each case leads to only 1 successful transmission of the infection. This implies constant incidence over time.if a greater proportion are immune, then incidence will decline.Oon this basis(R0-1)/R0 is known as the herd immunity threshold.
Example: If 80% of population is immune to a virus, Four out of every Five people who encounter some one with the disease wouldnot get sick (and wouldnot spread the disease any further). In this way ,the spread of infectious Diseases is kept under control. Depending how contagious an infection is,usually 70% to 90% of a population needs immunity to achieve Herd immunity.
Conclusion
The pandemic covid-19 has spread globally to more than 216 countries,territories,areas by causing more than 100 thousands of deaths.This pandemic led us to complete shutdown in terms of sevice,bussiness,economically and restrict ourselfs to safe guard from covid-19.But to what extend this shutdown is possible ? not for longer time it leads to economic,employement crisis and more than that global hunger deaths.The W.H.O estimate Basic reproduction rate (R0) is 1.4 to 2.5 which is higher than influenza virus.The studies across globally stating (R0) is higher than W.H.O estimations it would be in between 2.2 to 6.49. This basic reproduction rate the pandemic COVID-19 is going to infect millions of humans globally.Vaccination is the most preferrable method. vaccination against a viral infection is a administration of vaccine to an individual with the intention of immunizing that individual (or) boosting pre-existing immunity but the arrival time of vaccine for COVID -19 is estimated as 6 to 12 months.HERD IMMUNITY is a form of protection from an infectious disease that results when a large enough proportion of the population has become immune,conferring indirect protection from infection on individuals who are not themselves immune by safeguarding the humans with COMORBIDITIES.
REFERENCES
- Tu H, Tu S, Gao S, Shao A, Sheng J. The epidemiological and clinical features of COVID-19 and lessons from this global infectious public health event. J Infect. 2020;81:1-9.
- Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. SARS-COV2 and COVID-19-the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924.
- Wang L, `Wang Y, Ye D, Liu Q. A Review on 2019 novel corona virus (COVID-19) based on current evidence-Int J Antimicrob Agents. 2020;55:105948.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. clinical features of patients with 2019 novel corona virus in Wuhan, China. Lancet. 2020;395:497-506.
- Chan JF, To KK, Tse H, Jin DY, Yuen KY. Inter species transmission and emergence of novel viruses; lessons from bats and birds. Trends Microbiol. 2013;21:544-555.
- Cui J, Li F, Shi ZL. Origin and evolution of pathogenic corona virus. Nat Rev Microbiol.2019;17:181-192.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel corona virus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, et al. genome composition ad divergence of the novel Corona virus (2019-nCOV) originating in China. Cell host Microbe.2020;27:325-328.
- Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human corona virus-international weekly. Nature. 2013;495:251-254.
- Wan Y, Shang J, Graham R, Baric Rs, Li F. Receptor recognition by the novel corona virus from wuhan; an analysis based on decade-long structural studies of SARS corona virus-J Virol. 2020;94: e00127-20.
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of Angiotensin Converting Enzyme-2 (ACE-2) in SARS corona virus-induced lung injury. Nat Med. 2005;11:875-879.
- Wrapp D, Wang N, Corbett K, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCOV spike in the perfusion confirmation. Science. 2020;367:1260-1263.
- El-Aziz TM. Stockand JD. recent progress and challenges in drug development against COVID-19 corona virus (SARS-COV2)-an update on the status. Infect Genet Evol. 2020;83: 104327.
- Li H, Liu SM, Yu XH, Tang SL, Tang Ck. Corona virus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020;55:105951.
- Yeshwanth, BhanuPrasad R. The outbreak of corona virus (COIVD-19). International Journal of Medical and Health Research. 2020;6:79-81.
- Rothan HA, Byrareddy S. The epidemiology and pathogenesis of corona virus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433.
- Wu D, Wu T, Liu Q, Yang Z. The SARS-COV2 outbreak: what we know. Int J Infect Dis. 2020;94:44-48.
- Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT imaging features of 2019 novel corona virus (2019 nCOV). Radiology. 2020;295:202-207.
- Linda. J. Carter et al. Assay techniques and test development for COVID-19 diagnosis. ACS Cent Sci. 2020;6:591-605.
- Qiu G, Gai Z, Tao Y, Schmitt J, Kullak-Ublick GA, Wang J, et al. dual functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome corona virus-2 detection. ACS NANO. 2020;14: 5268–5277.
- Lu H. Drug treatment options for the 2019 new corona virus (2019 n-COV). Biosci Trends. 2020;14:69-71.
- Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic corona viruses. Sci Transl Med. 2017;9:eaal3653.
- Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical finding. Thorax. 2004;59:252-256.
- Zhang J, Xie B, Hashimoto K. Current Status of potential therapeutics candidates for the COVID-19 Crisis. Brain Behav Immun. 2020;87:59-73.
- Macdonald G (1957) The epidemology and control of malaria. london: Oxford University press.
- Roy M (1992) Anderson.Infectious diseases of humans:dynamics and control. Oxford university press.
- Liu Y, Gayle AA, Wilder-Smith A, Rocklov J. The reproductive number of COVID-19 is higher compared to SARS corona virus. J Travel Med. 2020;27: taaa021.
Citation: Yeshwanth S, Rajashekhar I, Rani I (2020) A Review on Covid-19 Pandemic Outbreak. J Microb Biochem Technol. 12:449 Doi: 10.35248/1948-5948.20.12.449
Copyright: © 2020 Yeshwanth S, et al. This is an open access article distributed under the term of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

