Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 4, Issue 1
A Randomized Trial to Evaluate the Efficacy and Safety of 1% Pimecrolimus Cream vs. 0.05% Clobetasol Propionate Cream for the Treatment of Childhood Vitiligo
Preeti Sharma1*, Amit Kumar1, Amit Ranjan2, Dhiraj Kumar1, Bidisha Roy3, Vikas Shankar3 and Ramawatar Singh32Department of Dermatology, PMCH Patna, India
3Department of Dermatology, NMCH Patna, India
Received: 06-Jun-2018 Published: 09-Jan-2019
Abstract
Objective: To assess the safety and efficacy of topical 1% Pimecrolimus cream vs. 0.05% clobetasol propionate cream for the treatment of childhood vitiligo.
Place of work: Department of Dermatology, Nalanda Medical College and Hospital, Patna, India.
Participants: In twenty-two children with vitiligo, two symmetrical lesions of about the same size and evolution time were selected. They were devoid of any topical or systemic therapy for two months prior to inclusion.
Interventions: Treatment of focal vitiligo with either topical 1% Pimecrolimus cream or 0.05% clobetasol propionate cream for a 3 month period.
Main outcome measures: The grade of repigmentation was evaluated by photographs at baseline and again at every 2 week visit. Characteristics of pigment, time of response, symptoms, telangiectasias, and atrophy were evaluated every 2 weeks.
Results: Eighteen (81.81%) of the 22 patients experienced some repigmentation. The mean percentage of repigmentation was 35.91% for Pimecrolimus and 40.45% for clobetasol. Lesions in 1 patients using clobetasol presented atrophy, and 2 lesions incurred telangiectasias, Pimecrolimus caused a burning sensation in 2 lesions.
Conclusions: Pimecrolimus 1% proved almost as effective as clobetasol propionate to restore skin color in lesions of vitiligo in children. Because it does not produce atrophy or other adverse effects, pimecrolimus 1% may be very useful for younger patients and for sensitive areas of the skin such as lips, eyelids, glans, and it should be considered in other skin disorders currently treated with topical steroids for prolonged periods.
Keywords
Childhood vitiligo; Pimecrolimus; Clobetasol; Comparative study
Introduction
Vitiligo is an acquired, primary, pigmentary disorder of skin and mucous membranes. It is characterised by circumscribed, white macules and patches often associated with leukotrichia as a result of loss of functioning melanocytes from the involved area. Several other studies have reported an incidence ranging from 0.1% to 1.3% in different parts of the globe [1,2]. Its prevalence in Indians is 0.46% [3]. Vitiligo has a special significance to patients in our country because depigmentation is more appreciable on darker skin type and it is also associated with lots of social stigma bringing lots of psychosocial impact to the patient suffering from vitiligo. The exact etiology of vitiligo is not known. Various theories regarding etiology have been propagated. These include autoimmune, cytotoxic, biochemical, oxidant, antioxidant, neural, and viral mechanisms. As with many disorders, it is likely that no single pathway is solely responsible but that multiple pathogenic mechanisms overlap to manifest disease in patients with vitiligo. Vitiligo usually starts in childhood or adolescence but may occur at any age. The disease affects subjects of either sex with maximum incidence between ages 10-30 (Table 1).
| S No | Age (year) |
Sex | Duration Of Disease | % of Area involvement | Treated Area | Repigmentation % Score (After 3 month) |
Adverse Effects | ||
|---|---|---|---|---|---|---|---|---|---|
| Pimecrolimus | Clobetasol | Pimecrolimus | Clobetasol | ||||||
| 1 | 3.5 | M | 4 M | <10% | Face | 3 | Burning | ||
| 2 | 4 | M | 2 Y 6 M | 10-49 % | Legs | 1 | |||
| 3 | 6 | F | 2 Y | <10% | Face | 3 | |||
| 4 | 5.5 | F | 1Y 6 M | 10-49% | Thorax | 2 | |||
| 5 | 11 | M | 3 Y 2 M | <10% | Hand | 0 | |||
| 6 | 13 | M | 2 y | <10% | Abdomen | 3 | |||
| 7 | 8 | M | 1Y 6 M | 10-49% | Ankle | 0 | |||
| 8 | 16 | M | 10Y | 10-49% | Axilla | 1 | Mild Burning | ||
| 9 | 9 | M | 4 Y | 10-49% | Forearm | 3 | |||
| 10 | 11 | F | 3 Y | 10-49% | Waist | 2 | |||
| 11 | 12 | M | 6 Y | 50-90% | Thorax | 1 | |||
| 12 | 13 | M | 3 Y | 10-49% | Shoulder | 0 | |||
| 13 | 08 | M | 3 Y | <10% | Leg | 3 | |||
| 14 | 09 | F | 6 M | 10-49% | Thorax | 2 | |||
| 15 | 4 | M | 9 M | <10% | LEG | 2 | |||
| 16 | 3 | F | 6 M | <10% | Face | 4 | Erythema | ||
| 17 | 6.5 | M | 1Y 3 Mo | <10% | Face | 3 | Erythema, Telangiectasia | ||
| 18 | 12.5 | F | 3 Y | 10-49% | Abdomen | 1 | |||
| 19 | 17 | M | 7 Y | 10-49% | Thorax | 2 | Atrophy | ||
| 20 | 12 | F | 6 M | <10% | Leg | 2 | |||
| 21 | 15 | M | 3 Y | 10-49% | Abdomen | 3 | Striae, Telangiectasia | ||
| 22 | 13 | M | 6 Y | 10-49% | Elbow | 0 | |||
Table 1: Table showing age, sex, comparison of repigmentation score and adverse effects between pimecrolimus and clobetasol.
On the basis of polymorphic distribution, extension and number of white patches, vitiligo is classified into following types: Generalised (Vulgaris, Acrofacial and Mixed), Universal and Localized (Focal, Segmental and Mucosal). Vitiligo is also classified as segmental and non-segmental types on the basis of distinctive clinical features and natural histories. Depending on its etiopathogenesis, vitiligo has also been classified as immune (Progressive), neural (segmental/ Dermatomal) [4].
The course of vitiligo cannot be predicted but it is mostly progressive. Spontaneous repigmentation may occur in a few people (10–20%), mainly in children, but this is not complete, occurs mostly on sun-exposed areas. The substantial disfigurement associated with vitiligo can cause serious emotional stress for the patient, which necessitates treatment. Treatment options for vitiligo are topical, physical and systemic and/or combined.
Topical treatment includes Corticosteroids High-potency fluorinated corticosteroid (e.g., clobetasol propionate ointment, 0.05%). Treatment can be gradually tapered to a lower potency corticosteroid (e.g., hydrocortisone butyrate cream, 0.1%). Calcineurin Inhibitor (Tacrolimus (0.03% and 0.1%), Pimecrolimus (1%). Vitamin D Derivatives: (Calcipotriol ointment (0.005%), Tacalcitol ointment (20μ/g)). Pseudocatalase and Basic Fibroblast Growth Factor.
Physical that includes Ultraviolet B (narrowband) (λ=311nm). Systemic Psorlen and Ultraviolet A light (PUVA). Excimer Laser (λ=308nm), Khellin with UVA Exposure (Khellin; a furanochrome isolated from the seeds of Ammi visnaga), Phenylalanine with UVA Exposure (5% aq. L-phenylalanine sol, 10% L-PA cream), Calcipotriol with PUVA therapy, Pseudocatalase and Ca chloride with short-term suberythemogenic UVB.
Systemic therapy includes Systemic Corticosteroids as minipulse therapy. Other drugs which have been tried are cyclophosphamide, azathioprine, levamisole, ACTH and oral corticosteroids.
Material and Methods
This prospective study of the comparative efficacy and safety of 1% Pimecrolimus vs 0.05% Clobetasol for the Treatment of Childhood focal Vitiligo was carried out in the Department of Skin, STD and Leprosy, Nalanda Medical College, Patna from Jan 2015 to Dec 2015.
Twenty two patients younger than 18 years (3-17 years) with vitiligo but otherwise healthy were selected for the study. All patients had disease duration more than 3 months. None of the patients had vitiligo therapy for 2 months prior to the study. Patients with mucosal and segmental vitiligo were excluded. A total of 22 patients were recruited; there were 15 boys and 7 girls. Only 3 patients had a positive family history. The mean age was 9.64 years (range, 3-17 years). The mean duration of disease was 2.93 years. The mean percentage of total area affected by vitiligo was 13.7 %. The localization of vitiligo was variable. 18 patients (81.81%) had duration of vitiligo more than 6 months (VIDA +3), and the other 4 had disease duration less than 6 months (Tables 2 and 3).
| Disease Activity | Vida Score |
|---|---|
| Active in past 6 weeks | +4 |
| Active in past 3 months | +3 |
| Active in past 6 months | +2 |
| Active in past 1 year | +1 |
| Stable for at least 1 year | 0 |
| Stable for at least 1 year and spontaneous repigmentation | -1 |
Table 2: Vitiligo Disease Activity.
| Total No. of Patients | 22 |
|---|---|
| Boys | 15 |
| Girls | 07 |
| Positive Family History | 03 |
Table 3: Total counts of patients for this study.
Proper clinical history of every selected patient that included patient’s name, age, sex, address, site and distribution of the disease, age at onset, family history, total area of depigmentation, and disease activity. Each selected patient of this study had given number from 1 to 22 serially according to their visit to OPD. Their informed consent was obtained from all patients, and the photograph of the lesion was taken. The study was approved by the local institutional review board.
Two lesions similar to each other in size and time of evolution were selected to apply either 1% pimecrolimus cream (PACROMA 1% CREAM Ajanta Pharma Ltd) or 0.05% clobetasol propionate cream (Tenovate Cream; GlaxoSmithKline) twice a day. The total amount of medication used ranged between 20 and 40g. Patients were evaluated every 2 weeks, and repigmentation and adverse effects were recorded. Color photographs of the lesions were taken at the beginning and end of the treatment period. The results were analysed visually by Clinician. The entire lesion was given a 0 score at the beginning of the study to indicate a baseline of no repigmentation. A second score was assigned at the end of the study to represent the level of repigmentation. The categories of repigmentation were as follows: no pigmentation (i.e.0%, Score 0), poor (1%-25%, Score 1-2), moderate (26%-50%, Score 3-4), good (51%-75%, Score 5-6), and Very Good (75%-90%, Score 7-8) and Excellent (>90%, Score 9-10) (Tables 4 and 5).
| Repigmentation | Area | Score |
|---|---|---|
| No Repigmrntation (At Beginning) | 0% | 0 |
| Poor Repigmentation | 1%-25% | 1 |
| Moderate Repigmentation | 26%-50% | 2 |
| Good repigmentaion | 51%-75% | 3 |
| Very Good Repigmentation | 76%-90% | 4 |
| Excellent Repigmentation | >90% | 5 |
Table 4: Vitiligo repigmentation scoring
| % Repigmentation | Score | No. of Patients (1% Pimecrolimus cream) | No. Of patients 0.05% clobetasol propionate cream |
|---|---|---|---|
| 0% | 0 | 2 (18.18%) | 2 (18.18%) |
| 1%-25% | 1 | 3 (27.27%) | 1 (9.09%) |
| 26%-50% | 2 | 3 (27.27%) | 4 (36.36%) |
| 51%-75% | 3 | 3 (27.27%) | 3 (27.27%) |
| 76%-90% | 4 | 0 (9.09%) | 1 (9.09%) |
| >90% | 5 | 0 (0%) | 0 (0%) |
Table 5: Comparison of repigmentation score in patients using 1% pimecrolimus vs 0.05% clobetasol propionate cream.
Results
Out of 22 patients, nineteen (86.36%) patients achieved some repigmentation with each treatment. Both medications were very well tolerated during the period of the study.
Evaluation of the clinical response showed that best effects of repigmentation were observed in the areas with greater density of hair follicles and face with both the drugs. No patient (0%) had excellent response with any medication. With 1% pimecrolimus cream, out of 11 patients, 2 (18.18%) patients showed no repigmentation (score-0); 3 (27.27%) patients showed poor repigmentation (score-1), 3 (27.27%) patients showed moderate repigmentation; 3 (27.27%) patients showed good repigmentation, Nil (0%) patient showed very good repigmentation and none showed excellent repigmentaion.
After 3 months of treatment with 0.05% clobetasol propionate cream; 2 (18.18%) patients showed no repigmentation; 1 (9.09%) patient showed poor re-pigmentaion, 4 (36.36%) patients showed moderate repigmentaion, 3 (27.22%) showed good repigmentaion, 1 (9.09%) patient showed very good repigmentaion and Nil showed excellent response (Figure 1).
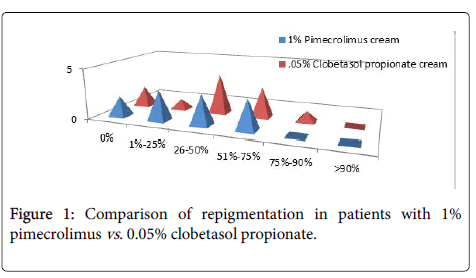
Figure 1. Comparison of repigmentation in patients with 1% pimecrolimus vs. 0.05% clobetasol propionate.
A significant negative correlation was detected between duration of disease and clinical response with both the treatment (Figure 2).

Figure 2. Photograph of a child treated with 1% pimecrolimus at baseline and after treatment at 12 weeks.
Side effects with pimecrolimus cream were marginal and transient noted in 2 patients in the form of mild irritation (that did not preclude continuation of therapy).
Erythema (2/11), atrophy (1/11) and telangiectasias (2/11), Striae (1/11) were noted after 12 weeks of treatment with 0.05% clobetasol propionate cream (Figure 3).
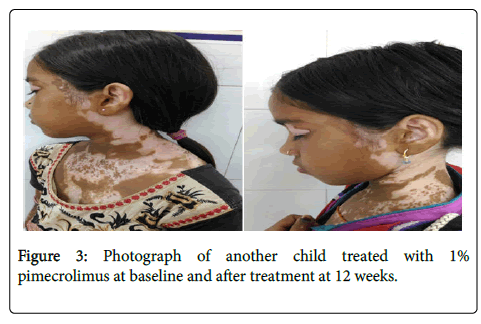
Figure 3. Photograph of another child treated with 1% pimecrolimus at baseline and after treatment at 12 weeks.
Discussion and Conclusion
As the pathogenesis of vitiligo is still not understood, there are number of different treatments. Among them, topical steroids and narrowband ultraviolet B monotherapy are the most common current treatments for localized and generalized vitiligo, respectively. Cosmetic improvement can be achieved by camouflage products and selftanning dyes.
Even after so many years, there is no ideal treatment of vitiligo as the exact pathogenesis of the disease is still elusive. Topical corticosteroids are used as first line therapy for localized vitiligo in children and adults. The response rate in children was 45% and 57% according to Halder [5] and Cho et al. [6] respectively. Adverse effects after prolonged treatment have led to search of new alternatives. Topical calcineurin inhibitors (Pimecrolimus and Tacrolimus), has been advocated considering the autoimmune hypothesis of vitiligo pathogenesis. Topical calcineurin inhibitors and potent corticosteroids are both well tolerated and effectively used as pigment inducer. Topical potent corticosteroids, especially potent ones are associated with cutaneous atrophy, telangiectasias and these side-effects are seen more in children who already have soft and thin skin and in this scenario, topical calcineurin inhibitors offer the better option.
In our present study, we compared treatment with pimecrolimus and clobetasol propionate for repigmentation in vitiligo patch in different areas of the body in patients’ age group less than 18 years. The advantage of pimecrolimus compared with conventional corticosteroids is that it is more selective in its mechanism and do not induce skin atrophy. Pimecrolimus has added advantage of not causing skin atrophy especially in areas where skin is already thin, thus pimecrolimus can be safely used in flexural areas and face where use of topical steroids will lead to atrophy and striae formation.
In present study, pimecrolimus is almost as effective as clobetasol propionate but side effects are more common with topical clobetasol cream. More studies are required to support this conclusion.
REFERENCES
- Bertolino AP, Freeberg IM (1987) Hereditary syndromes with noncicatricial alopecia. In: Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF, (eds) Dermatology in internal medicine (3rd ed.). New York, McGraw-Hill education, USA. pp: 636–639.
- Hunter MH, Carek PJ (2003) Evaluation and treatment of women with hirsutism. Am Fam Physician J 67: 2565–2572.
- Howitz J, Brodthagen H, Schwartz M, Thomsen K (1977) Prevalence of vitiligo. Epidemiological survey on the Isle of Bornholm, Denmark. Arch Dermatol 113: 47-52.
- Yilmaz M, Biri A, Karakoc A, Törüner F, Bingöl B, et al. (2005) The effects of rosiglitazone and metformin on insulin resistance and serum androgen levels in obese and lean patients with polycystic ovary syndrome. J Endocrinol Invest 28: 1003–1008.
- Halder RM. Childhood Vitiligo. Clin Dematol 15: 899-906.
- Cho S, Kang HC, Hahm JH (2000) Characteristic of Vitiligo in Korean Children. Pediatr Dermatol 17: 189-93.
Citation: Sharma P, Kumar A, Kumar D, Singh R, Sinha R, et al. (2019) A Randomized Trial to Evaluate the Efficacy and Saftey of 1% Pimecrolimus Cream vs. 0.05% Clobetasol Propionate Cream for the Treatment of Childhood Vitiligo. J Dermatitis 4:117.
Copyright: © 2019 Sharma P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.