Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- Google Scholar
- Quality Open Access Market
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 9, Issue 3
A New Predictive Model for the Time of Cardiac Arrest in-ICU Palliative Care Patients: A Single Center Retrospective Cohort Study
Shunsuke Takaki*Received: 02-May-2023, Manuscript No. JPMME-23-21191; Editor assigned: 04-May-2023, Pre QC No. JPMME-23-21191 (PQ); Reviewed: 19-May-2023, QC No. JPMME-23-21191; Revised: 26-May-2023, Manuscript No. JPMME-23-21191 (R); Published: 05-Jun-2023, DOI: 10.35248/2684-1320.23.9.211
Abstract
Objective: The aim of this study was to develop a predictive model for the time of cardiac arrest in the palliative care setting in the intensive care unit.
Design: We retrospectively collected patients’ data in intensive care unit between 2010 and 2016. Vital signs were collected until cardiac arrest occurred and Systolic Blood Pressure (SBP) was continuously recorded when it was less than 80 mmHg. We hypothesized that a breakdown of the autonomic nervous system was associated with the time of cardiac arrest. We aimed develop predictive model for determine a time at 120 minutes before cardiac arrest. We used the Shock Index (SI) defined as heart rate divided by SBP as the ratio variable to identify an end-of-life phase.
Results: A total of 4,330 patients were admitted to the ICU and 19 patients out of them were included to this study. We developed a prediction model by using SI and SBP: predicted SI=0.995+(6.931-0.995) e-0.035 x SBP with AUC 0.650 (0.512 to 0.788). The disparity between the actual and predicted heart rate was -10 bpm (49.9%, sensitivity; 75.8%, specificity; and likelihood ratio, 2.06). In the validation set, sensitivity was 52.7%, specificity was 79.8%, positive predictive value was 35.7%, negative predictive value was 88.8%, and likelihood ratio was 2.61.
Conclusion: Our new prediction model estimates the time to death 120 minutes before cardiac arrest occurs based on the information of fluctuation of shock index.
Keywords
Death prediction; Palliative care; Cardiac arrest; Autonomic dysfunction; Shock index
Introduction
The super-aging of the population has become one of the most significant social issues in Japan. In this society, palliative care plays a key role in helping patients reach the end of their lives happily. In the End Of Life (EOL) care setting, the physician’s ability to predict the time of death as more accurately as possible is an important research topic since it may allow family members to spend the last moments of patient’s life with the patient, which leads to improving family satisfaction. A systematic review study to determine a particular healthcare professional who is able to better-prognosticate the length of survival in all disciplines finds the difficulty for any clinicians to accurately predict the prognosis in palliative care [1]. Another systematic review study defines the independent factors that most consistently contribute to time to death after withdrawal of life-sustaining care [2]. A prospective cohort study identifies predictors of death in patients who are suspected to be infected under the emergency department environment [3].
However, a previous study to develop a predictive model by using the University of Wisconsin Donation After Cardiac Death Evaluation tool has shown relatively poor sensitivity and specificity to identify the exact time of death, and thus, further research is required to develop a reliable one [4,5]. In the EOL care setting, unexpected sudden or prolonged death can affect the terminal care process involving the family and patient, and cause difficulties in the process of organ donation after circulatory death. This is another important reason to develop a more accurate predictive model for the time to death in palliative and EOL care settings. Although many studies have developed predictive models by using patient physiological and physical variables, those tools are not enough sensitive to predicting the time to death [2]. Our hypothesis was that the lower sensitivity and specificity could be attributed to the patient dataset utilized for model development. The majority of previous studies used a single dataset from a single patient at any given time in their predictive models. Using a single dataset per patient may be insufficient as individual patients have various cardiac function, autonomic nervous system and backgrounds.
Several studies indicate the association of Heart Rate Variability (HRV) between all cause of mortality, cardiovascular events, and death prediction so that in this study, HRV was measured every 30 minutes [6-8]. They focused on the correlation between cardiac autonomic dysfunction and the prediction for the time to death.
Although a previous study examined the variation of vital signs as a predictive factor for the last day of terminally ill patients, they used patient’s vitals twice per day to predict the time to death, since Bruera, et al. concluded routinely monitoring vitals in patients at the EOL care was not supportive to predicting death [8,9]. Any previous studies did not use continuously measured vitals to predict the time to death, and hence, we focused on physiological parameters collected every one minute in the palliative care context we defined. We hypothesized the discrete variations of heart rate and blood pressure are caused that autonomic nervous system dysfunction occurs prior to cardiac arrest. Therefore, identifying these discrete variations is considered a key point to predict the time to death in the palliative care setting. The aim of this study was to develop a new predictive model with included individual fluctuation patterns in the continuously measured vitals during deterioration in the palliative care context. The new model will allow patients who are imminently dying and their family members to share the last moments together before cardiac arrest occurs, as well as healthcare professionals to use intensive care beds more efficiently in cases of in-ICU cardiac arrest.
Materials and Methods
Study design
We retrospectively collected data from patients who were admitted to the Intensive Care Unit (ICU) between 2010 and 2016. The definition of subjects in this study was those who were unresponsive to treatment for the primary disease and had hemodynamic instability but were not transferred to the palliative care hospital ward and finally diagnosed with cardiac arrest in the ICU. Patients’ vitals were continuously measured until cardiac arrest by inserting an arterial line. Patients’ physiological information was extracted from the physiological monitor (Nihon Kohden central monitoring system, CNS-6210R) every one minute. The physiological parameters included Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Mean Blood Pressure (MBP), heart rate, respiratory rate, temperature, and oxygen saturation. They were collected until the patient’s heart rate was recorded as 0 beats per minute from hospital admission to ICU admission. During this study, we defined a palliative care situation as when patients were unresponsive to treatment for primary disease and had hemodynamic instability and identified vitals at the point of deterioration under the defined clinical condition. A SBP of less than 80 (<80) mmHg for 10 minutes was defined as a worsening of the patient’s condition, and it was assumed that the EOL process had begun. Vital signs recorded from the physiological monitoring system typically contain artifacts due to a number of factors, such as blood test sampling from arterial lines, nursing care, restless behaviors at the annulation attachment site, and so forth. Therefore, we manually checked them to evaluate whether they represented the patients’ conditions accurately. We then excluded data which was considered inaccurate due to artifacts. We assigned to each vital data a remaining time to death which gradually decreased until patients died, in order words, patient’s heart rate became 0 bpm. Also, we collected as baseline demographics age, height, body weight, diagnosis, and length of ICU stay.
We involved a two-step approach to predict time to death. In the first step, we predicted a time at 120 minutes before cardiac arrest by using our predictive model and then applied physiological data. Chiang, et al.’s study has found that cardiovascular autonomic functions are associated with time to death by using heart rate variability on admission [7]. Moridani, et al. have reported that cardiac autonomic dysfunction is associated with remaining time to death [8]. Therefore, we focused on this system failure, which causes an imbalance between heart rate and blood pressure, and used this information, namely heart rate and blood pressure, to develop a predictive model for the time at 120 minutes before cardiac arrest. Our predictive model is developed by using a nonlinear regression method. We considered the dispersion from the non-linear regression model as the deterioration to cardiac arrest.
Statistical methods
This retrospective observational study was approved by Yokohama City University Hospital's institutional review board (Ref: B170200037) and registered with the University Medical Information Network (UMIN ID: 000028958). Therefore, patient informed consent was waived. Descriptive patient data is expressed as median values and interquartile range. Vital signs were collected from a physiological central monitoring system and divided into two groups: 1) a Volatile Set (VS), dataset extracted during the period over 120 minutes prior to cardiac arrest; 2) a Terminal Set (TS), dataset collected within 120 minutes before cardiac arrest. According to a scaling law for the training set to validation set ratio, we randomly selected 80% of patient vital data for training set and 20% for the validation set both in the VS and TS [10]. By using the training set, we developed a new predictive model for death within 120 minutes before cardiac arrest. We conducted non-linear regression analysis for the predictive model with an exponential decay model. In this analysis, we used Systolic Blood Pressure (SBP) and the Shock Index (SI), which is obtained from the formula of heart rate divided by SBP, as the ratio variable. The predicted heart rates calculated from the non-linear regression model were compared with the actual heart rates. During the model development phase, we determined the threshold for disparity between the predicted heart rate and the actual heart rate.
We determined the threshold by using the nonparametric t test, the log rank test, and Receiver Operating Characteristic (ROC) curve for the area under the curve. For two-tailed tests, p-value less than 0.05 were considered statistically significant. We performed statistical analyses with Prism 6 for Mac OS X6.9b (GraphPad Software, San Diego, California) and R 2.13.0 statistical software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
A total of 4,330 patients were admitted to the ICU between 2010 and 2017 and 32 patients were diagnosed with in-ICU cardiac arrest. 13 out of them were excluded due to data insufficiency and age criterion (less than 18 years old). Finally, 19 patients were included in this study and 12,050 datasets consisted of several vitals were recorded. 12,038 datasets were registered with this study but 12 were manually excluded where the presence of artifacts or errors during measurement was apparent at the discretion of the clinician. We assigned 9,915 out of 12,038 datasets (82.3%) to VS and 2,111 to TS. 80% of each dataset is used as the training set, and we selected randomly 7,932 datasets in VS and 1,689 datasets in TS, respectively. Furthermore, in the training set, 6,243 randomly selected VS datasets were used for non-linear regression analysis, and 1,689 datasets from each of VS and TS were used to determine threshold. We then allocated the rest of each data set (20%), 1,983 from VS and 422 from TS, to the validation set (Figure 1). Demographic characteristics of subjects were expressed in median and interquartile range: Age (64 years; 53 to 75 years old); sex (men 15/19; 79%), length of ICU stay (3 days; 1 to 8 days), length of hospital stay (7 days; 3 to 23 days) (Table 1). The diagnoses were categorized as follows: 5 cardiac surgical (26%), 4 surgical (21%), 5 medical (26%), and 5 cancer (26%) [11].
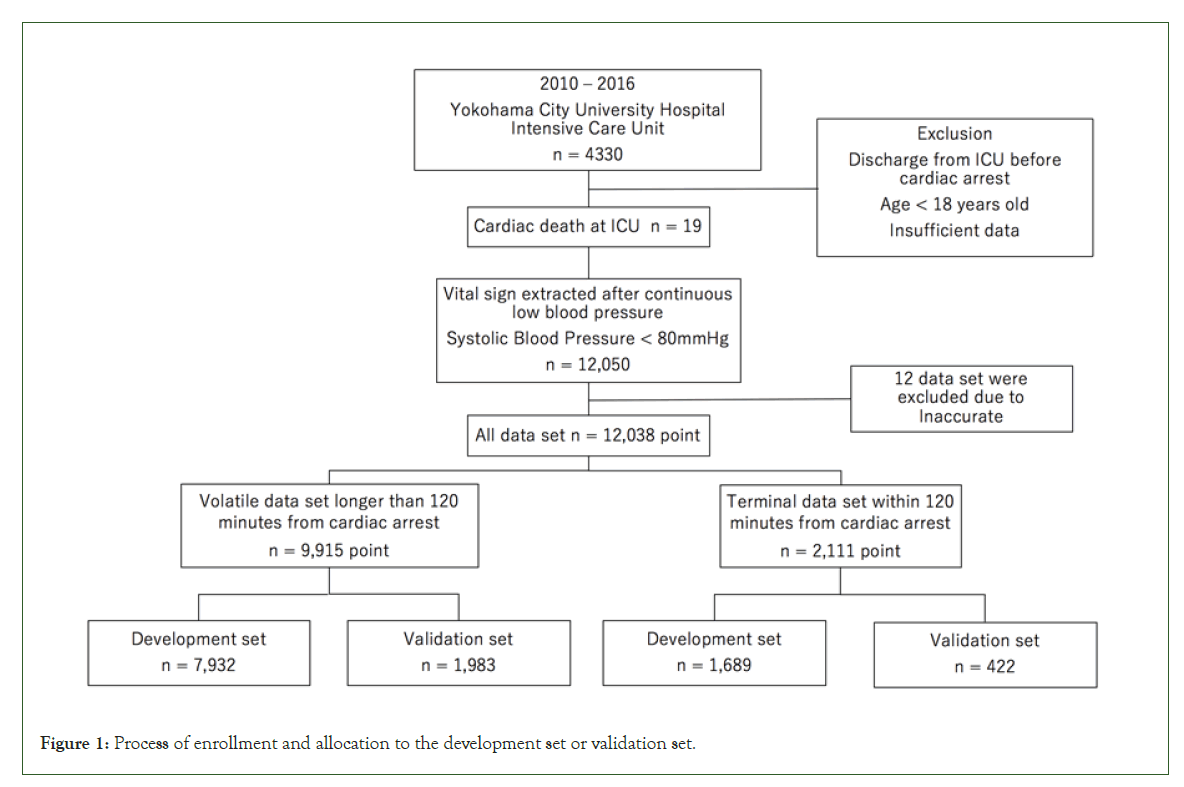
Figure 1: Process of enrollment and allocation to the development set or validation set.
| Factor | n = 19 |
|---|---|
| Age (yr) | 63 (53 to 75) |
| Sex (male, %) | 15/19, 79% |
| Duration of ICU stay (days) | 3 (1 to 8) |
| Duration of Hospital stay (days) | 7 (3 to 23) |
| Diagnosis (n, %) | Cardiac 5/19, 26.3% |
| Surgical 4/19, 21.1% | |
| Medical 5/19, 26.3% | |
| Cancer 5/19, 26.3% | |
| Note: Values are expressed with median and interquartile range and number of patients and ratio. | |
Table 1: Demographic characteristics of patients who included in this study.
Outcome data
Median and interquartile range of remaining time to death from the moment when blood pressure was recorded <80 mmHg was 279 minutes (126 to 864 minutes). A curve-fitting for the association between shock index and SBP was shown by non-linear regression with an exponential decay model, which is commonly used in the natural science studies [12]. From 6,243 training set, an exponential decay was derived by using the following equation:
Y = Plateau + (Y0 − Plateau)e−KX
The parameters of this equation were the followings: Y0=6.931 (6.638 to 7.224), plateau=0.995 (0.926 to 1.063), K=0.035 (0.327 to 0.037). Then, the following equation was derived.
Predicted shock index = 0.995 + (6.931− 0.995) e−0.035×systolic blood pressure
R square is 0.699 (Figure 2).
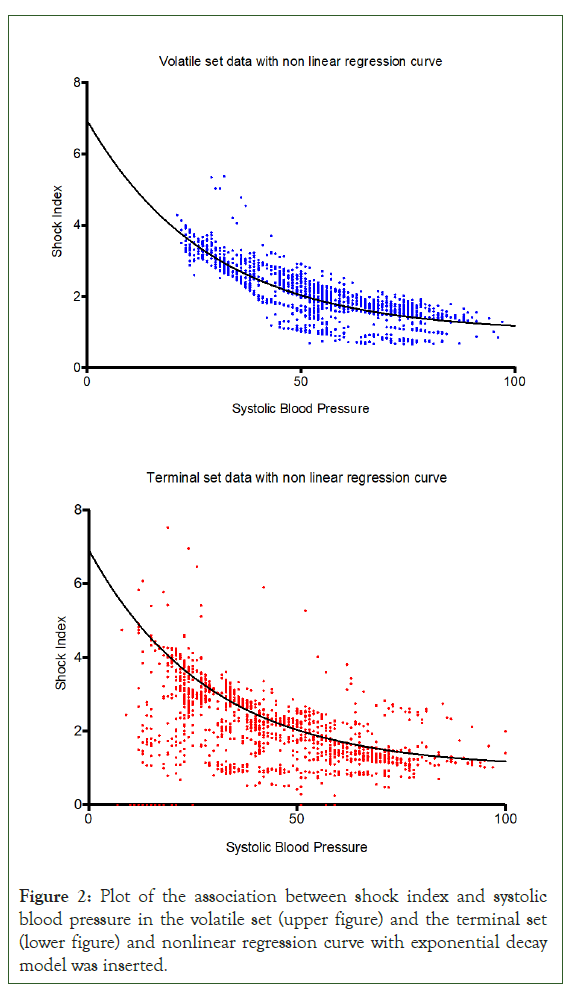
Figure 2: Plot of the association between shock index and systolic blood pressure in the volatile set (upper figure) and the terminal set (lower figure) and nonlinear regression curve with exponential decay model was inserted.
Median and interquartile range of the disparity in the two datasets are 1.90 (1.1 to 2.9) for VS and -9.8 (-10.9 to -8.5) for TS. Area under the ROC of the predictive model by using the training set was 0.650 (0.512 to 0.788), p-value<0.001, and the threshold of disparity between actual and predicted heart rate was 10 bpm with sensitivity of 49.9%, specificity of 75.8%, and likelihood ratio of 2.06 (Figure 3).
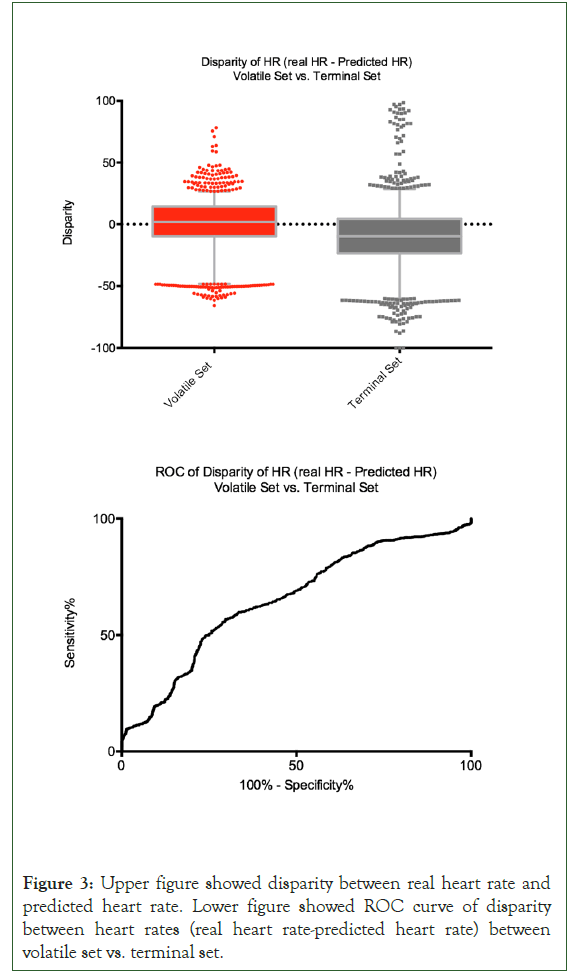
Figure 3: Upper figure showed disparity between real heart rate and predicted heart rate. Lower figure showed ROC curve of disparity between heart rates (real heart rate-predicted heart rate) between volatile set vs. terminal set.
In the validation set, the prognostic ability of the model for cardiac arrest developed by using the training set was assessed. The results were the followings: Sensitivity 52.7% (47.8 to 57.6), specificity 79.8% (78.0 to 81.6), positive predictive value 35.7% (31.9 to 39.6), and negative predictive value 88.8% (87.2 to 90.3), and likelihood ratio 2.61.
Discussion
Our predictive model could estimate the remaining time to death within 120 minutes before cardiac arrest under the palliative care situation with a valid prognostic ability (Table 2). In detail, our predictive model showed 52.7% sensitivity, 79.8% specificity, 35.7% positive predictive value, and 88.8% negative predictive value. The model accuracy was superior to the predictive models in the previous studies: 42% sensitivity and 76% specificity by Lewis, et al., 39% sensitivity and 96% specificity by Coleman, et al., and 79% sensitivity and 63% specificity by de Vita, et al [4,5,13]. The novelty in this study is that we used continuously measured vital signs which were record every one minute in order to develop our predictive model. Brieva, et al. reported that the evaluation by intensivists is the clinical standard to predict time to death after withdrawal of cardiorespiratory support, and the evaluation was done at an arbitrary timing [14]. In this study, we repeatedly predicted the time of cardiac arrest by taking advantage of data from physiological monitor, and this continuity is one of other advantages against previous studies. In the past researches, different parameters have been reported to predict cardiac arrest, which include not only physiological data but also a blood test, a past medical history, and a background [3,15,16]. Our predictive model is a computer-based, and therefore, automatically calculated by using SBP and heart rate, which makes our model easily adoptable in any existing clinical workflows. Furthermore, although we developed this model in the ICU setting, the necessary parameters are commonly used standard variables so that it can be applied not only to the critical care settings but also chronic care settings.
| Factor | Estimate | Std. Error | t-value | p-value |
|---|---|---|---|---|
| (Intercept) | 12.34 (2.25-22.43) | 5.14 | 2.4 | 0.017 |
| Heart Rate | 0.34 (0.28-0.4) | 0.03 | 11.57 | < 0.001 |
| Respiratory Rate | -0.25 -(0.36-0.14) | 0.06 | -4.37 | < 0.001 |
| Systolic Blood Pressure | 0.28 (0.18- 0.37) | 0.05 | 5.81 | < 0.001 |
| Sex. Male.1, Female.0. | -0.60 (-4.56-3.37) | 2.02 | -0.3 | 0.77 |
| Age | 0.18 (0.06-0.29) | 0.06 | 2.9 | 0.004 |
Note: Residual standard error: 28.87 on 1287 degrees of freedom Multiple R-squared: 0.3167, Adjusted R-squared: 0.314; F-Statistic: 119.3 on 5 and 1287 degrees of freedom, p-value: <2.2e-16. |
||||
Table 2: Residual time to cardiac arrest with linear regression model analyzed with vital data within 120 minutes from cardiac arrest in the development set.
Another key finding is the association between the breakdown of the autonomic nervous system and the time to death. Several studies reported the analysis of HRV, which was determined by the interaction between the sympathetic and parasympathetic autonomic nervous system, is a useful predictor of death [6-8]. In our predictive model, systolic blood pressure and heart rate were used as predictive parameters which represented this interaction. However, HRV should be carefully interpreted, and the control of breathing is crucial for accurate interpretation [17]. Billman found that HRV was associated with mechanical events due to the change in thoracic pressure and cardiac filling pressure during respiration [17]. Furthermore, Chiang, et al. reported that cardiac arrhythmia and major cardiac surgery affect HRV [7]. In this study, indeed, we focused on the balance between blood pressure and heart rate, not on HRV though we collected the data. In a controlled cardiac nervous system, blood pressure and heart rate are linked by the sympathetic and parasympathetic nervous system [18]. In the future study, we will develop a predictive model by targeting individuals and determining an equation based on the pattern of autonomic nervous system per patient. Our unique continuous measurement of vital signs can repeatedly predict the imbalance between the sympathetic and parasympathetic nervous systems, making the model higher prognostic ability.
In the palliative care setting, it is of significant importance to take enough time for patients to stay with their family members before postmortem changes occur. Predicting the time to death 120 minutes before cardiac arrest occurs allows most family members to reach the patient’s bed before the patient dying, especially in the urban setting. Also, prognostication of death may also help healthcare professionals to plan for a better patient care and communicate earlier with family members.
As mentioned, the accurate prognostication of the remaining time to cardiac arrest would be valuable for healthcare professionals in that they can allow the patient’s family members to say their final goodbyes to the patient who is imminently dying. However, healthcare professionals should always discuss with the family carefully whether or not they want to know how much time remains before cardiac arrest. Individuals have different attitudes toward death and may be influenced by religious beliefs. Therefore, in clinical practice, it is important to recognize that formulas may interfere with the dying process.
Conclusion
Our new prediction model estimates the time to death 120 minutes before cardiac arrest occurs based on the information of fluctuation of shock index. Our findings were derived by analyzing physiological data measured continuously in the ICU. Therefore, it may have limited generalizability in other clinical settings. The development of a new monitoring system will allow any hospital wards to collect a patient’s vitals continuously, which will then enable to apply our predictive model to anywhere without borders.
Limitations
There are several limitations to this research. Firstly, the sample size was small with 19 subjects, even though a large number of vitals were recorded per patient. The reason for this sample size is that in our hospital patients diagnosed as terminal, which was defined as being unresponsive to treatment for primary disease and having hemodynamic instability, were transferred to a different hospital ward to allow them to spend the last time with their family members. Therefore, in general, very few patients go into cardiac arrest while staying in the ICU.
Secondly, although we conducted statistical analyses by using repeatedly measured vitals from different patients as one cluster, they might include bias due that an individual dataset had many data collected from the same patient. To solve them, a future study should increase sample size of patients. Thirdly, although we defined 120 minutes as the threshold for allocating data to the two groups of VS and TS, the predictive model for 120 minutes before cardiac arrest may not be enough for a patient family to reach the hospital, especially in the rural setting. It might be, indeed, more appropriate to use a different timeframe to differentiate these two groups. Lastly, our predictive model required continuous invasive blood pressure and heart rate monitoring. Besides the ICU settings, non-invasive blood pressure monitoring is typically used to measure patient’s blood pressure so that future studies targeting non-ICU patients should select alternative variables, measurement interval, and monitoring devices.
This new predictive model that estimates the remaining time to cardiac arrest had a reasonable prognostic ability. In the palliative and EOL care settings, this model can predictably identify the malfunction of the autonomic nervous system by continuously tracking the variations in heart rate and systolic blood pressure before cardiac arrest. Further research will be required to examine individual patterns of fluctuations in hemodynamic parameters in the palliative care situation prior to death. Furthermore, it is important to develop a predictive model that is implemented in noninvasive ways and can prognosticate the remaining time to cardiac arrest in different clinical settings.
Acknowledgement
We appreciate all the research staffs and the healthcare providers who supported our research.
References
- White N, Reid F, Harris A, Harries P, Stone P. A systematic review of predictions of survival in palliative care: how accurate are clinicians and who are the experts? PloS one. 2016;11(8):e0161407.
[Crossref] [Google Scholar] [Pub Med]
- Munshi L, Dhanani S, Shemie SD, Hornby L, Gore G, Shahin J. Predicting time to death after withdrawal of life-sustaining therapy. Intensive Care Med. 2015;41:1014-1028.
[Crossref] [Google Scholar] [Pub Med]
- Shapiro NI, Wolfe RE, Moore RB, Smith E, Burdick E, Bates DW. Mortality in Emergency Department Sepsis (MEDS) score: A prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31(3):670-675.
[Crossref] [Google Scholar] [Pub Med]
- Lewis J, Peltier J, Nelson H, Snyder W, Schneider K, Steinberger D, et al. Development of the University of Wisconsin donation after cardiac death evaluation tool. Prog Transplant. 2003;13(4):265-273.
[Crossref] [Google Scholar] [Pub Med]
- Coleman NL, Crowfoot E, Brieva JL. Prediction of death after withdrawal of life-sustaining treatments. Crit Care Resusc. 2008;10(4):278-284.
- Fang SC, Wu YL, Tsai PS. Heart rate variability and risk of all-cause death and cardiovascular events in patients with cardiovascular disease: A meta-analysis of cohort studies. Biol Res Nurs. 2020;22(1):45-56.
[Crossref] [Google Scholar] [Pub Med]
- Chiang JK, Koo M, Kuo TB, Fu CH. Association between cardiovascular autonomic functions and time to death in patients with terminal hepatocellular carcinoma. J Pain Symptom Manage. 2010;39(4):673-679.
[Crossref] [Google Scholar] [Pub Med]
- Karimi Moridani M, Setarehdan SK, Motie Nasrabadi A, Hajinasrollah E. Non-linear feature extraction from HRV signal for mortality prediction of ICU cardiovascular patient. J Med Eng Technol. 2016;40(3):87-98.
[Crossref] [Google Scholar] [Pub Med]
- Bruera S, Chisholm G, Dos Santos R, Crovador C, Bruera E, Hui D. Variations in vital signs in the last days of life in patients with advanced cancer. J Pain Symptom Manage. 2014;48(4):510-517.
[Crossref] [Google Scholar] [Pub Med]
- Guyon I. A scaling law for the validation-set training-set size ratio. AT&T Bell Lab. 1997:1-11.
- Akaike H. Information theory and an extension of the maximum likelihood principle. In2nd International Symposium of Information Theory. Akademia Kiado, Budapest. 1973:267-281.
- Leike A. Demonstration of the exponential decay law using beer froth. Eur J Phys. 2001;23(1):21.
[Crossref] [Google Scholar]
- DeVita MA, Brooks MM, Zawistowski C, Rudich S, Daly B, Chaitin E. Donors after cardiac Death: Validation of Identification Criteria (DVIC) study for predictors of rapid death. Am J Transplant. 2008;8(2):432-441.
[Crossref] [Google Scholar] [Pub Med]
- Brieva J, Coleman N, Lacey J, Harrigan P, Lewin TJ, Carter GL. Prediction of death in less than 60 minutes following withdrawal of cardiorespiratory support in ICUs. Crit Care Med. 2013;41(12):2677-2687.
[Crossref] [Google Scholar] [Pub Med]
- Morita T, Tsunoda J, Inoue S, Chihara S. Improved accuracy of physicians' survival prediction for terminally ill cancer patients using the Palliative Prognostic Index. Palliat Med. 2001;15(5):419-424.
[Crossref] [Google Scholar] [Pub Med]
- Rady MY, Verheijde JL. Prediction of time to death after terminal withdrawal of life-support in non-heartbeating organ donation: Unaccounted variables and window of opportunity. Crit Care Med. 2012;40(3):986-988.
[Crossref] [Google Scholar] [Pub Med]
- Billman GE. Heart rate variability-a historical perspective. Front Physiol. 2011;2:86.
[Crossref] [Google Scholar] [Pub Med]
- Matsukawa T, Sugiyama Y, Mano T. Age-related changes in baroreflex control of heart rate and sympathetic nerve activity in healthy humans. J Auton Nerv Syst. 1996;60(3):209-212.
[Crossref] [Google Scholar] [Pub Med]
Citation: Takaki S (2023) A New Predictive Model for the Time of Cardiac Arrest in-ICU Palliative Care Patients: A Single Center Retrospective Cohort Study. J Pain Manage Med. 9:211
Copyright: © 2023 Takaki S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.