Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 17, Issue 2
A Healthy Participant Study to Evaluate the Bioequivalence of Form H and Form II Tablets of the Smallpox Antiviral Brincidofovir
David M Cassie1*, Vanja Komlenovic1, James McCarty2, Jing Tian2 and Kevin Yeo32Emergent BioSolutions, 300 Professional Drive, Suite 400, Gaithersburg, Maryland, 20879, USA
3Emergent BioSolutions UK Ltd., Building 3, Chiswick Park, 566 Chiswick High Road, London, W4 5AY, England
Received: 16-Apr-2025, Manuscript No. JBB-25-28921; Editor assigned: 21-Apr-2025, Pre QC No. JBB-25-28921 (PQ); Reviewed: 05-May-2025, QC No. JBB-25-28921; Revised: 12-May-2025, Manuscript No. JBB-25-28921 (R); Published: 19-May-2025, DOI: 10.35248/0975-0851.25.17.626
Abstract
Background: Brincidofovir (BCV) is approved in the US and Canada for the treatment of human smallpox disease in adults and children, including neonates. In long-term storage, the commercially available BCV morphic Form II is slowly converted to a hydrated morphic Form H, which is more stable under ambient conditions. The purpose of this study (NCT05935917) was to compare the Bioequivalence (BE) of the morphic Forms II and H.
Methods: This was a Phase 1, open-label, randomized, two-period crossover study completed in healthy adults randomized to receive either a single 100 mg BCV Form II or Form H tablet and followed for 14 days after each dose to assess Pharmacokinetics (PK) and safety. The primary PK endpoints for demonstration of BE were Cmax , AUClast , and AUCinf of BCV in plasma. BE was declared if the 90% Confidence Interval (CI) for the true ratio of test to reference geometric means fell entirely within the range of 0.80 to 1.25 for these endpoints.
Results: Forty-four (44) healthy subjects were enrolled, received Form II and Form H treatments, and completed all study visits and safety assessments. For the primary endpoints AUCinf, AUClast , and Cmax, the 90% CIs of the geometric mean ratios for Cmax, AUCinf and AUClast for Form H and Form II fell within the predefined range, thereby demonstrating BE. No new safety signals were identified.
Conclusion: Natural conversion of BCV from Form II to Form H over the shelf life does not change the safety or PK profile of BCV in healthy participants.
Keywords
Brincidofovir; BCV; Bioequivalence; Smallpox; Variola virus
Introduction
Smallpox (variola virus infection), one of the most devastating diseases of human history, was successfully eradicated by vaccination in 1977 [1,2]. However, variola virus remains a potential biological weapon and the risk of a smallpox outbreak necessitates preparedness [2,3].
Brincidofovir (BCV) is a lipid-modified, acyclic nucleotide DNA polymerase inhibitor providing intracellular delivery of the active antiviral Cidofovir-Diphosphate (CDV-PP) [4,5]. The antiviral activity of BCV has been characterized in vitro in cell culture systems and in vivo in multiple animal models. In cell culture assays, BCV is active against double-stranded DNA viruses, including orthopoxviruses (variola, vaccinia, monkeypox, camelpox, cowpox), polyomaviruses, human herpes viruses, human papillomaviruses, and adenoviruses [4,6,7]. In vivo, the antiviral activity of BCV has been characterized in animal models of orthopoxvirus, cytomegalovirus, herpes simplex virus, varicella zoster virus, and adenovirus infection. A dose of BCV was identified that significantly reduced viral burden in each model and protection against mortality was seen in those using lethal inoculums. BCV is approved in the United States and Canada for the treatment of human smallpox disease in adults and children, including neonates [8,9]. BCV is made available to clinicians from the U.S. Strategic National Stockpile for treatment of mpox who request and obtain an FDA-authorized single-patient emergency use Investigational New Drug (e-IND) application [10-12].
The lipid moiety of BCV determines its distribution and Pharmacokinetic (PK) properties, differentiating it from CDV. The lipid conjugate facilitates cell entry of BCV, where it is cleaved to yield CDV, which is further phosphorylated to the active DNA polymerase inhibitor, Cidofovir Diphosphate (CDV-PP) by intracellular anabolic kinases [13-14]. This process results in lower circulating plasma concentrations of CDV. CDV-PP exerts its antiviral effect by acting as a potent alternate substrate inhibitor of viral DNA synthesis [15].
Brincidofovir, chemical name “Phosphonic acid, [[(S)-2-(4-amino- 2-oxo-1(2H)-pyrimidinyl)-1-(hydroxymethyl) ethoxy]methyl]mono[3- (hexadecyloxy)propyl] ester” is an active pharmaceutical ingredient in approved drug products, tablets and suspension. BCV drug substance has three distinct physical forms identified as Form I, Form II, and Form H. Both Form I and Form II convert to the hydrate (Form H) following ingestion, or upon exposure to high humidity or aqueous media. Form H is the most thermodynamically stable form under most ambient conditions. In long-term storage, the commercially available BCV Form II is slowly converted to Form H.
The purpose of this study was to demonstrate the Bioequivalence (BE) of the morphic Form II (commercial) and the morphic Form H tablets. The data generated in this study showed bioequivalence between two BCV forms indicating the same expectations for tablets over the shelf life, and the potential for enabling extension from the current 48 months shelf life. Extending the shelf life is critical for the maintenance of Medical Countermeasure (MCM) stockpiles, and to support the availability of this antiviral to combat potential outbreaks.
Methods
Ethical conduct of the study
The study protocol and informed consent form were reviewed and approved by an independent ethics committee (Advarra Inc. Institutional Review Board, Columbia, MD, USA). The study was conducted at Altasciences Clinical Kansas, Inc, Overland Park, Kansas, USA in accordance with ethical principles that originate from the Declaration of Helsinki and that are consistent with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline for Good Clinical Practice (GCP) in effect at the time of study conduct.
Volunteer population
Enrollment was open to sterilized males or females (or postmenopausal), 18–70 years of age, with a body mass index (BMI) of 18–32 kg/m2 and a minimum body weight of = 50 kg, and able and willing to sign informed consent. Subjects were overtly healthy as determined by medical evaluation and judgement of the investigator, including medical history, physical examination, laboratory tests, and Electrocardiogram (ECG) at screening and Day -1. Any history of chronic liver disease or hepatic impairment, Gilbert’s syndrome, hematological disorders, positive drug screen or positive serology for Hepatitis B Virus (HBV), Hepatitis C Virus (HCV) or Human Immunodeficiency Virus (HIV), or serious psychiatric illness, was exclusionary.
Study design
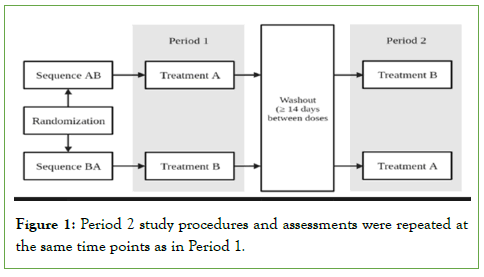
This was a Phase 1, open-label, randomized, two-period, crossover study completed in healthy subjects (NCT05935917). The primary objectives of the study were: (a) to evaluate the BE of Form H (treatment A; test tablet) and Form II (treatment B; reference tablet) when administered under fasting conditions, (b) to characterize plasma BCV PK following single doses, and (c) to evaluate the safety of BCV following single 100 mg doses of Form H and Form II tablets. The study evaluated the safety and PK of BCV following administration of two 100 mg single doses over two treatment periods (Table 1).
| Sequence | Period 1 | Period 2 |
|---|---|---|
| AB (n = 22) | Treatment A: 100 mg BCV Form H tablet | Treatment B: 100 mg BCV Form II tablet |
| BA (n = 22) | Treatment B: 100 mg BCV Form II tablet | Treatment A: 100 mg BCV Form H tablet |
Table 1: Study sequences
Drug Administration
Participants were screened and consented over a 28-day period with inpatient admission on Day -1. On Day 1 of Period 1 participants were randomized to receive either a single 100 mg BCV Form II or Form H tablet following fasting. Participants were discharged on Day 3 and outpatient visits occurred on Days 4, 5, 7, 11 and 14, where vital signs, labs, concomitant medications and Adverse Events (AEs) were assessed. To avoid any carry-over effect, a wash-out of 14 days was planned between drug administrations, corresponding to more than 10 times the expected half-life of BCV. After a period of =14 days, subjects were re-admitted to the clinic and crossed over to alternate treatment in Period 2. All Period 2 study procedures and assessments were repeated at the same time points as in Period 1 (Figure 1).
Figure 1: Period 2 study procedures and assessments were repeated at the same time points as in Period 1.
PK blood sampling and analysis
Twenty-one (21) blood samples were collected from each subject at each period for BCV PK measurements at pre dose (within 15 minutes prior to dosing) and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 8, 10, 12, 16, 24, 36-, 48-, 72-, and 96-hours post dose. The concentration analysis of BCV in plasma was performed by PPD laboratories using a LC-MS/MS assay validated from 1–750 ng/ mL.
Statistical Analysis
Bioequivalence analysis: The analysis populations pre-defined for this study included the: (1) Safety Population: all participants who received at least one dose of Form H or Form II tablet, and (2) Pharmacokinetics Population: participants who received at least one dose of Form H or Form II tablet and had at least one quantifiable post-dose concentration of BCV without protocol deviations. The PK parameters determined were Maximum plasma Concentration (Cmax), Area Under the plasma concentration-time Curve from time zero to time of last measurable plasma concentration (AUClast), area under the plasma concentration curve from time 0 to infinity (AUCinf), half-life (t1/2), and time to peak (maximum) concentration (tmax) of BCV in plasma. The primary PK endpoints of the study for demonstration of BE were Cmax, AUClast, and AUCinf of BCV in plasma.
This study was designed to demonstrate BE of the morphic Form II (commercial) and Form H tablets. For assessment of BE, the null hypothesis was that the two tablets of BCV were not bioequivalent, i.e., the true ratio of the geometric mean for Form H (test treatment) to the geometric mean of the commercial tablet Form II (reference treatment), for BCV plasma AUC and Cmax, was either less than 0.80 or greater than 1.25. The alternative hypothesis was that the tablets are bioequivalent, i.e., the true ratio of the geometric mean for the test tablet (Form H) to the geometric mean of the reference tablet (Form II) for BCV plasma AUC and Cmax was between 0.8 and 1.25. For each PK parameter designated as a primary endpoint, a two one-sided t-test procedure with a=0.05 for each one-sided test was used to test this set of hypotheses (Schuirmann 1987). Bioequivalence was declared if the 90% Confidence Interval (CI) for the true ratio of test to reference geometric means fell entirely within the range of 0.80 to 1.25 for all primary PK parameters.
The sample size was based on the following assumptions:
1) An intra-participant Coefficient of Variation (CV) of approximately 25%. (The estimate is approximately the largest CV of BCV PK parameters AUCinf, and Cmax from a previous BE study).
2) The true ratio of means for BCV PK parameters AUCinf and Cmax for the Form H tablet vs the Form II tablet is between 0.95 and 1.05.
3) A two-period crossover design. With these assumptions, a sample size of 38 participants would have approximately 90% power for a BE test of means comparing the Form H tablet vs the Form II tablet using two one-sided tests at a 5% significance level. To allow for a 15% dropout rate, 44 participants were enrolled to study interventions so that at least 38 evaluable participants would complete the study.
Safety analysis: All safety analyses were reported using the Safety Population. The primary safety endpoints were clinical and laboratory safety parameters including AEs, absolute values and changes over time of hematology and clinical chemistry, and vital signs. Safety data were analyzed descriptively using frequencies of events or continuous statistical summaries by BCV treatment. Treatment- Emergent AEs (TEAEs), severe AEs, AEs leading to withdrawal, and serious AEs (SAEs) were summarized by treatment, System Organ Class (SOC) and Preferred Term (PT) using the Medical Dictionary for Regulatory Activities (MedDRA). Laboratory values and vital sign values and corresponding changes from baseline as appropriate were summarized by treatment, study day and planned time. Laboratory analytes for which participants had results outside the normal range were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) grades (Version 5). Maximum grade post dose for each participant in each treatment was calculated and summarized.
Results
Bioequivalence
Forty-four (44) healthy subjects were enrolled, received Form II and Form H treatments, and completed all study visits and safety assessments. The Safety and Pharmacokinetic populations both contained 44 participants. The participants had a mean age of 53.7 years, 90.9% were female, 84.1% were non-Hispanic/Latino, and 63.6% were White. This study included a skewed population of primarily older age non-hispanic white women. Per the US and Canadian prescribing information, no clinically meaningful differences in the PK of BCV have been observed based on age, sex or race. The mean body weight, height and BMI were 73.4 kg, 165.2 cm and 26.8 kg/m2, respectively (Table 2). Demographics were balanced between the two groups.
| Treatment AB (N=22) | Treatment BA (N=22) | Overall (N=44) | ||
|---|---|---|---|---|
| Age (years) | Mean (SD) | 54.1 (8.14) | 53.3 (10.22) | 53.7 (9.14) |
| Min, Max | 38, 66 | 33, 68 | 33, 68 | |
| Sex [n(%)] | Female | 21 (95.5) | 19 (86.4) | 40 (90.9) |
| Male | 1 (4.5) | 3 (13.6) | 4 (9.1) | |
| Ethnicity [n(%)] | Not Hispanic/Latino | 18 (81.8) | 19 (86.4) | 37 (84.1) |
| Hispanic/Latino | 1 (4.5) | 3 (13.6) | 4 (9.1) | |
| Unknown | 3 (13.6) | 0 | 3 (6.8) | |
| Race [n(%)] | White | 14 (63.6) | 14 (63.6) | 28 (63.6) |
| Black/African American | 5 (22.7) | 7 (31.8) | 12 (27.3) | |
| American Indian/Alaska Native | 1 (4.5) | 1 (4.5) | 2 (4.5) | |
| Multiple | 2 (9.1) | 0 | 2 (4.5) | |
| Weight (kg) | Mean (SD) | 70.76 (10.45) | 76.00 (12.07) | 73.38 (11.47) |
| Min, Max | 53.5, 92.6 | 54.5, 96.7 | 53.5, 96.7 | |
| Height (cm) | Mean (SD) | 163.84 (7.20) | 166.64 (7.66) | 165.24 (7.48) |
| Min, Max | 152.0, 182.0 | 155.0, 184.0 | 152.0, 184.0 | |
| Body Mass Index (kg/m2) | Mean (SD) | 26.28 (2.66) | 27.32 (3.53) | 26.80 (3.13) |
| Min, Max | 22.2, 31.3 | 20.8, 31.8 | 20.8, 31.8 | |
Table 2: Demographic and baseline characteristics – Safety population
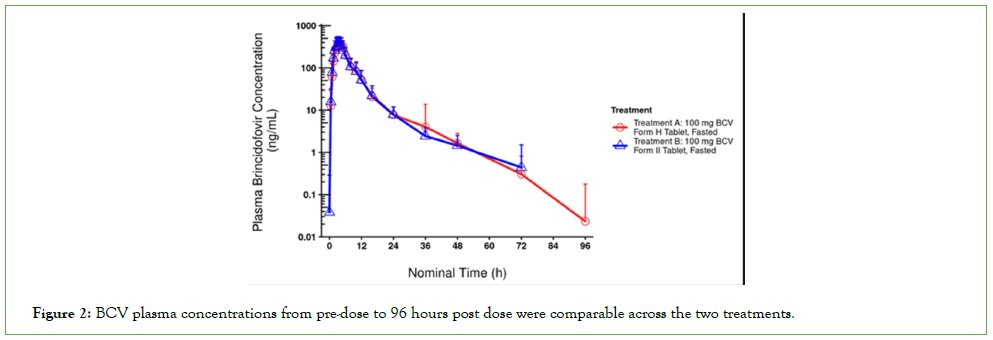
Summaries of plasma BCV concentration data included data for all participants and Noncompartmental Analyses (NCA) were performed using actual sampling times. BCV plasma concentrations from pre-dose to 96 hours post dose were comparable across the two treatments (Figure 2). From the NCA, post dose Cmax geometric mean values were comparable across treatments and the tmax ranged from 3–4 hours. The half-life and all other pharmacokinetic parameters were also comparable across the two treatments (Table 3).
Figure 2: BCV plasma concentrations from pre-dose to 96 hours post dose were comparable across the two treatments.
| Cmax (ng/mL) | Tmax (h) | T1/2 (h) | AUCinf (h*ng/mL) | AUClast (h*ng/mL) | ||
|---|---|---|---|---|---|---|
| 100 mg Form H Tablet (Treatment A; test) | Median | 408 | 4 | 10.8 | 2340 | 2280 |
| Geometric mean (CV%) | 411 (38.4) | - | 12.0 (61.2) | 2270 (42.8) | 2210 (41.4) | |
| 100 mg Form II Tablet (Treatment B; reference) | Median | 442 | 3.1 | 9.62 | 2140 | 2080 |
| Geometric mean (CV%) | 402 (44.6) | - | 10.7 (58.8) | 2170 (46.5) | 2150 (44.9) | |
Cmax = Maximum serum concentration, Tmax = time to peak (maximum) plasma concentration, T1/2 = half-life, AUCinf = Area Under the plasma concentration Curve from time 0 to infinity, AUClast = Area Under the plasma concentration-time Curve from time zero to time of last measurable plasma concentration, CV = Coefficient of Variation
Table 3: Pharmacokinetic parameters by treatment
For the primary endpoint AUCinf, AUClast, and Cmax, the 90% CIs of the geometric mean ratios for Cmax, AUCinf and AUClast for Form H and Form II fell within the predefined range of 0.80 to 1.25, thereby demonstrating BE (Table 4). The intra-subject CVs were very similar and lower than the projected 25% assumed for the power calculation.
| Parameter | Treatment A/B ratios of Geometric LCM (%) | Treatment A/B Geometric LCM ratio 90% CI (0.80, 1.25) | Intra-subject CV% |
|---|---|---|---|
| AUCinf (h*ng/mL) | 104 | 95.35, 112.36 | 20.6 |
| AUClast (h*ng/mL) | 103 | 95.54, 110.55 | 20.3 |
| Cmax (ng/mL) | 103 | 94.93, 111.18 | 22.1 |
AUCinf = Area Under the plasma concentration Curve from time 0 to infinity, AUClast = Area Under the plasma concentration-time Curve from time zero to time of last measurable plasma concentration, Cmax = Maximum serum Concentration, CI = Confidence Intervals, LSM = Least Squares Mean, CV = Coefficient of Variation
Table 4: Bioequivalence assessment for plasma BCV PK parameters
Safety
Both forms of BCV were well tolerated and the incidence of drug-related TEAEs was comparable for Form II (27.3%) and Form H (22.7%) participants. The majority were Grade 1 (mild), 86.4% and 71.4% for Form H and Form II, respectively, and the TEAEs were comparable across treatments. The most frequently reported TEAEs were headaches (9.1% for both Form H and Form II), GI disorders (9.1% for both Form H and Form II), and anemia (6.8% for Form H and 9.1% for Form II) (Table 5). Rates of gastrointestinal drug-related TEAEs were comparable to those documented in prior BE studies and noted in the Prescribing Information for TEMBEXA® (brincidofovir) [8, 9].
| System organ class MedDRA preferred term | BCV form H (Treatment-A) (N=44) n (%) | BCV form II (Treatment-B) (N=44) n (%) |
|---|---|---|
| Blood and lymphatic system disorders | 3 (6.8) | 4 (9.1) |
| Anaemia | 3 (6.8) | 4 (9.1) |
| Gastrointestinal disorders | 4 (9.1) | 4 (9.1) |
| Diarrhea | 2 (4.5) | 2 (4.5) |
| Nausea | 2 (4.5) | 1 (2.3) |
| Injury, poisoning, and procedural complications | 2 (4.5) | 0 |
| Contusion | 2 (4.5) | 0 |
| Investigations | 0 | 3 (6.8) |
| Blood creatine phosphokinase increased | 0 | 2 (4.5) |
| Musculoskeletal and connective tissue disorders | 3 (6.8) | 0 |
| Pain in extremity | 2 (4.5) | 0 |
| Nervous system disorders | 4 (9.1) | 4 (9.1) |
| Headache | 4 (9.1) | 4 (9.1) |
| Skin and subcutaneous tissue disorders | 3 (6.8) | 0 |
| Dermatitis contact | 2 (4.5) | 0 |
Abbreviations: BCV = Brincidofovir; MedDRA = Medical Dictionary for Regulatory Activities; PT = Preferred Term; SOC = System Organ Class. n = number of subjects; N = number of subjects in Safety Population. MedDRA v26.0.
Note: Each TEAE was counted only once for each participant within each MedDRA SOC and PT
Table 5: Summary of treatment emergent adverse events experienced by at least two participants
One participant with an elevated Creatine Phosphokinase (CPK) level at baseline experienced a Grade 3 (severe) TEAE of blood CPK increased after administration of Form II, which returned to baseline. Twelve participants experienced clinically significant lab values (blood CPK elevated, hyperkalemia, anemia, neutrophil count decreased and proteinuria) that were transient, and all returned to baseline levels. Three participants had Alanine Aminotransferase (ALT) elevations that were deemed not clinically significant, were transient and returned to baseline levels during the study or soon after the follow-up period. No participants experienced Grade 4 TEAEs and there were no SAEs reported.
Discussion
BCV has been developed and approved for the treatment of smallpox [17, 18]. It has a broad spectrum of activity against orthopoxviruses and is more active in vitro than CDV [7, 9, 20]. BCV reduced mortality in mice and rabbits infected with lethal doses of ectromelia and rabbitpox viruses and was approved for the treatment of variola virus infection through the FDA “Animal Rule” (21 CFR 314.600) [8, 9]. BCV is made available from the U.S. Strategic National Stockpile for treatment of mpox to clinicians who request and obtain an FDA-authorized single-patient emergency use Investigational New Drug (e-IND) application for treatment of mpox [11, 12].
The clinical development program for BCV began in 2006 and included prospective safety and efficacy trials in 1732 subjects. These included 650 and 950 subjects in Phase 1 and 2/3 studies, respectively, of oral BCV. The Phase I studies of oral and IV BCV included healthy subjects, and those with renal and hepatic impairment, whereas the Phase 2/3 studies included Hematopoietic Cell Transplant (HCT) and solid organ transplant patients. In addition, approximately 2000 patients have been treated for double-stranded DNA virus infections, mostly cytomegalovirus and adenovirus, through an expanded access protocol and via individual eIND mechanisms in the US and equivalent regulatory mechanisms ex-US.
The safety profile of BCV is well characterized based on the treatment of >3700 subjects as part of controlled clinical trials and expanded access programs and has not been studied in patients with smallpox disease as it was approved under the Animal Rule pathway [8, 9]. The most common AEs include diarrhea, nausea and vomiting. In clinical studies, elevations of Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) were documented in both ill and healthy populations, but these elevations were typically asymptomatic, predictable, and resolved after discontinuation of BCV, indicating a lack of significant liver injury. BCV has consistently demonstrated less nephrotoxicity than CDV, which is attributed to the lower peak plasma CDV concentrations after BCV administration than after IV CDV administration. In addition, unlike CDV, BCV is not a substrate for the human Organic Anion Transporter (OAT) 1 and therefore is not concentrated in the renal tubule [21]. A Phase 3, placebo-controlled trial of BCV in allogeneic Hematopoietic Cell Transplantation (HCT) recipients demonstrated an imbalance in the incidence of Graft Versus Host Disease (GVHD) and week 24 mortality in the BCV group following 14 weeks of treatment, leading to a black box warning [22]. Graft versus host disease would not be expected in the population receiving the approved 2-week course of treatment for smallpox. Additional warnings based solely on animal data include the potential for carcinogenesis, mutagenesis and impaired male fertility [16].
The current approved BCV formulations are 100 mg tablets and a 10 mg/mL oral suspension. The approved dosage for treatment of smallpox disease is 200 mg (2 x 100 mg tablets or 20 mL oral suspension for patients who cannot swallow tablets) once weekly for 2 weeks for adult and pediatric patients weighing 48 kg or above. For patients weighing 10 kg to less than 48 kg, 4 mg/kg of oral suspension is taken once weekly for 2 weeks. For pediatric patients weighing <10 kg, 6 mg/kg of oral suspension is taken once weekly for 2 weeks.
In this crossover study conducted to compare the BE of two forms of BCV, the administration of 100 mg of Form H and Form II tablets to healthy participants was safe and well tolerated. Demographics were comparable to prior BE studies at a similar dose level. The three primary endpoints, Cmax, AUClast, and AUCinf of BCV in plasma, were all met and BE of the morphic Form II and Form H was established. There were no significant safety findings and TEAEs were comparable across treatments, to prior BE studies, and to the expected GI and hepatic related adverse reactions in the Prescribing Information [8, 9]. All increases in laboratory values were transient and returned to baseline levels. The observed incidence of anemia noted here was not seen in previous BE studies and in this trial was attributed to the larger volumes of blood drawn for BCV plasma levels and safety laboratory testing [19].
Bioequivalence was declared for Form H and Form II as the 90% CIs of the geometric mean ratio fell entirely in the range of 0.80 to 1.25 for plasma BCV Cmax, AUCinf and AUClast.
Conversion of BCV Form II to Form H upon exposure to humidity in tablets over the shelf life will not change the safety or PK profile [20]. No impact on bioavailability between tablets with Form II and Form H is demonstrated. Hence, the dosing regimens with tablets containing Form II or Form H, or varying levels of two forms can be considered equivalent as per approved Prescribing Information in the United States (USPI) for TEMBEXA®.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Sources of Funding
This project has been supported in whole or in part with federal funds from the Department of Health and Human Services; Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority, under contract number 75A50122C00047. The findings and conclusions in this presentation have not been formally disseminated by the Department of Health and Human Services and should not be construed to represent any agency determination or policy.
References
- The Global Eradication of Smallpox. Final Report of the Global Certification of Smallpox Eradication. Geneva, 1979. Geneva, WHO, 1980.
- Breman JG. Smallpox. J Infect Dis. 2021;224:S379.
[Crossref] [Google Scholar] [PubMed]
- Petersen BW, Damon IK, Pertowski CA, Meaney-Delman D, Guarnizo JT, Beigi RH, et al. Clinical guidance for smallpox vaccine use in a postevent vaccination program. MMWR Recomm Rep. 2015;64:1-26.
[Google Scholar] [PubMed]
- Siegrist EA, Sassine J. Antivirals with activity against mpox: A clinically oriented review. Clin Infect Dis. 2023;76(1):155-164.
[Crossref] [Google Scholar] [PubMed]
- Hostetler KY. Synthesis and early development of hexadecyloxypropylcidofovir: An oral antipoxvirus nucleoside phosphonate. Viruses. 2010:2213–2225.
[Crossref] [Google Scholar] [PubMed]
- Prevost J, Sloan A, Deschambault Y, Tailor N, Tierney K, Azaransky K, et al. Treatment efficacy of cidofovir and brincidofovir against clade II Monkeypox virus isolates. Antiviral Res. 2024;231:105995.
[Crossref] [Google Scholar] [PubMed]
- Imran M, Alshammari MK, Arora MK, Dubey AK, Das SS, Kamal M, et al. Oral brincidofovir therapy for monkeypox outbreak: A focused review on the therapeutic potential, clinical studies, patent literature, and prospects. Biomedicines. 2023;11(2):278.
[Crossref] [Google Scholar] [PubMed]
- Tembexa. Prescribing Information. 2024.
- Health Canada. TEMBEXA (brincidofovir) Product Monograph.2023.
- World Health Organization. Smallpox and mpox (orthopoxviruses) vaccines. Weekly epidemiological record 2024;34:429-456.
- Rao AK. Interim clinical treatment considerations for severe manifestations of mpox - United States, February 2023. MMWR. MMWR Morb Mortal Wkly Rep. 2023;72.
[Crossref] [Google Scholar] [PubMed]
- FDA's role in mpox preparedness and response, and information about mpox. 2024.
- Aldern KA, Ciesla SL, Winegarden KL, Hostetler KY. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-14C] cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol Pharmacol. 2003;63(3):678-681.
[Crossref] [Google Scholar] [PubMed]
- Cihlar T, Chen MS. Identification of enzymes catalyzing two-step phosphorylation of cidofovir and the effect of cytomegalovirus infection on their activities in host cells. Mol Pharmacol. 1996;50(6):1502-1510.
[Crossref] [Google Scholar] [PubMed]
- Hostetler KY. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: Current state of the art. Antiviral Res. 2009;82(2):84-98.
[Crossref] [Google Scholar] [PubMed]
- Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15:657-680.
[Crossref] [Google Scholar] [PubMed]
- Lanier R, Trost L, Tippin T, Lampert B, Robertson A, Foster S, et al. Development of CMX001 for the treatment of poxvirus infections. Viruses. 2010;2(12):2740.
[Crossref] [Google Scholar] [PubMed]
- Chan-Tack K, Harrington P, Bensman T, Choi SY, Donaldson E, O'Rear J, et al. Benefit-risk assessment for brincidofovir for the treatment of smallpox: US Food and Drug Administration's Evaluation. Antiviral research. 2021;195:105182.
[Crossref] [Google Scholar] [PubMed]
- Olson VA, Smith SK, Foster S, Li Y, Lanier ER, et al. In vitro efficacy of brincidofovir against variola virus. Antimicrob Agents Chemother. 2014;58(9):5570-5571.
[Crossref] [Google Scholar] [PubMed]
- Florescu DF, Keck MA. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev Anti Infect Ther. 2014;12(10):1171-1178.
[Crossref] [Google Scholar] [PubMed]
- Tippin TK, Morrison ME, Brundage TM, Momméja-Marin H. Brincidofovir is not a substrate for the human organic anion transporter 1: A mechanistic explanation for the lack of nephrotoxicity observed in clinical studies. Ther Drug Monit. 2016;38(6):777-786.
[Crossref] [Google Scholar] [PubMed]
- Marty FM, Winston DJ, Chemaly RF, Mullane KM, Shore TB, Papanicolaou GA, et al. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(2):369-381.
[Crossref] [Google Scholar] [PubMed]
Citation: Cassie D, Komlenovic V, McCarty J, Tian J, Yeo K. (2025). A Healthy Participant Study to Evaluate the Bioequivalence of Form H and Form II Tablets of the Smallpox Antiviral Brincidofovir. J Bioequiv Availab.17:626.
Copyright: © 2025 Cassie D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.